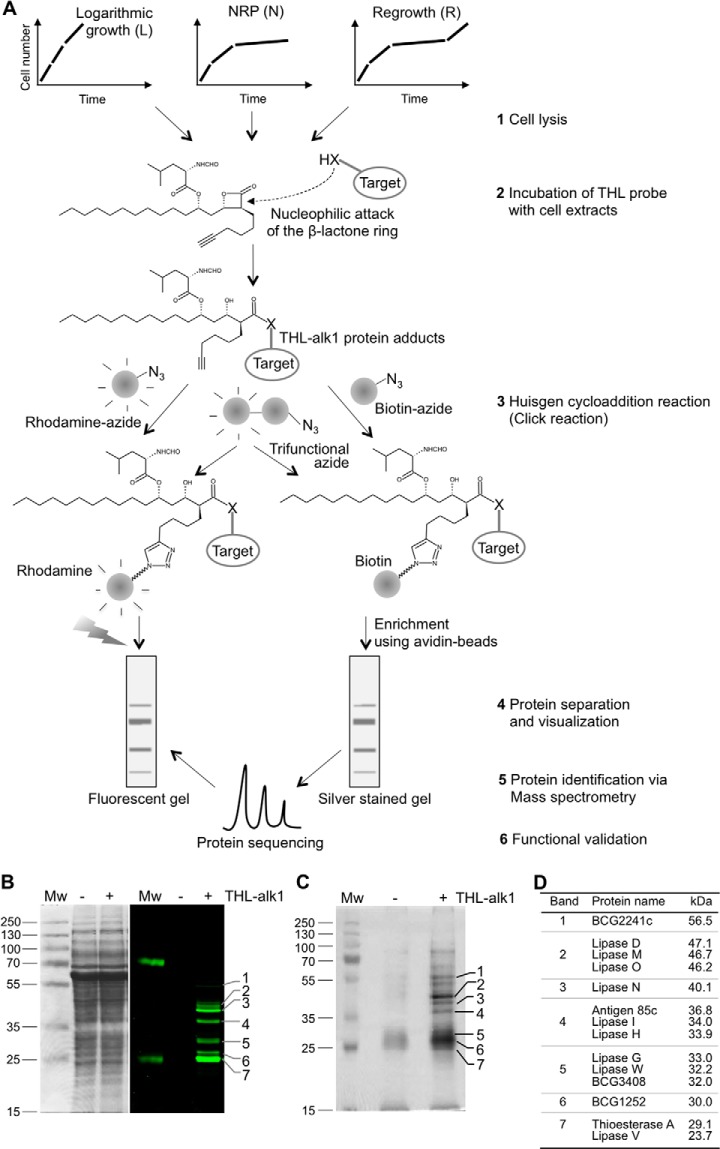
Fig. 1.
THL target spectrum in M. bovis BCG largely comprises lipid esterases. A, Schematic representation of the workflow and steps involved in identification of THL targets. M. bovis BCG was grown in three different physiological states in vitro, cells lysed by sonication (step 1) and total cell extracts incubated with THL-alk1 (step 2). The alkyne moiety of THL-alk1 was then used to either tag (via the Huisgen cycloaddition reaction) protein-THL adducts with rhodamine-azide or biotin-azide or trifunctional-azide (step 3). Whole cell lysates (in the case of rhodamine tagged adducts) or enriched fractions (in the case of biotin tagged adducts) were separated by SDS-PAGE (step 4, B, C). Protein identities (obtained by sequencing of peptides in enriched fractions via mass spectrometry, step 5, C) were compared with visualized protein patterns (step 4) to derive the THL target list (D) from which TesA (migrating with band 7) and LipH (migrating with band 4) were further validated (step 6). B, Mycobacterial cell lysates incubated with THL-alk1 or DMSO as a control were tagged to a rhodamine-azide dye by the Huisgen cycloaddition reaction. Equal amounts of proteins were separated on SDS-PAGE and visualized by Coomassie staining (left panel) or in-gel fluorescence (right panel). C, THL-alk1 bound targets were enriched using biotin-avidin affinity chromatography. Enriched fractions were separated on SDS-PAGE and stained with silver. The bands indicated on the right side were excised and subjected to tandem MS analysis. D, THL targets in M. bovis BCG total cell extracts derived from logarithmically growing cultures. Only proteins found consistently in at least 2 out of 3 three replicate experiments, not present in control incubations (DMSO in the absence of THL-alk1), and, with matching, co-migrating fluorescent targets (B), were included in this list.