Fig. 3.
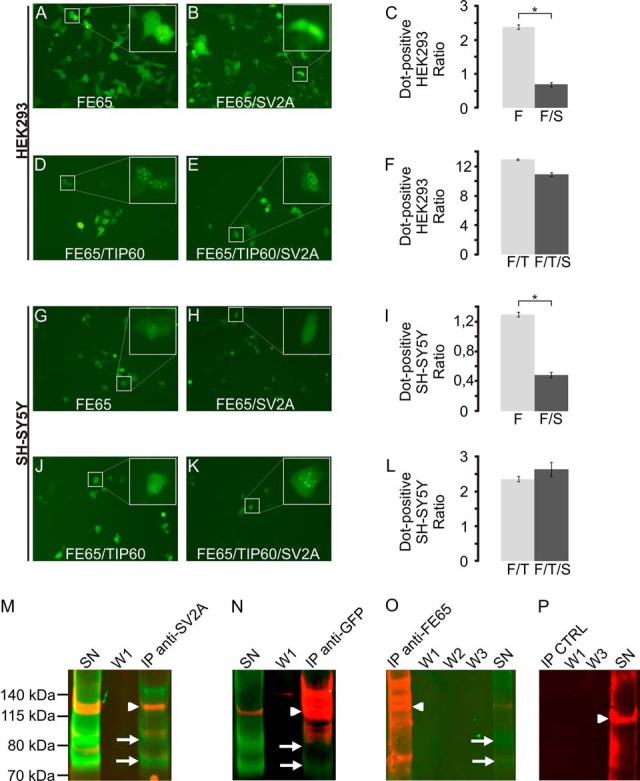
SV2A modulates the localization of FE65 and interacts with the adapter protein in co-immunoprecipitation assays. A–C, co-transfection of SV2A significantly modified the localization of FE65 in HEK293 cells, but not if TIP60 was co-transfected as well (D–F). Similar results were obtained with co-transfection experiments in SH-SY5Y (G–L). Co-immunoprecipitation assays validated the interaction of SV2A with FE65. In SV2A precipitates (M), both FE65 (arrowhead) and SV2A (arrows, two isoforms) could be identified. The co-immunoprecipitation supernatant (SN) still contained both proteins, whereas no signal was evident in the first washing step (W1). Conversely, immunoprecipitation of FE65-EGFP using an anti-GFP antibody (N) revealed the co-immunoprecipitation of SV2A (arrow). Immunoprecipitation using an anti-FE65 WW antibody (O) did not co-precipitate SV2A. However, SV2A was present in the immunoprecipitation supernatant. Control experiments using beads without antibody (P) showed no SV2A or FE65 signal.
