Fig. 1.
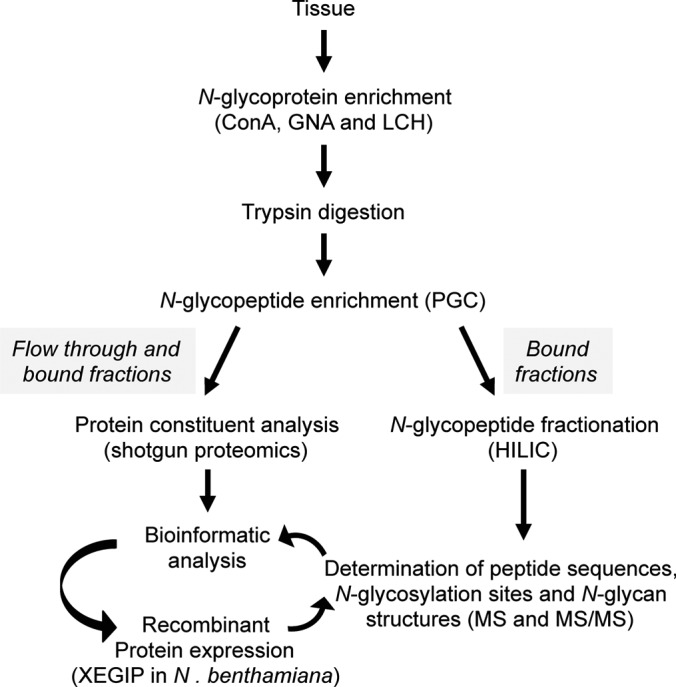
Experimental workflow used in this study. N-glycoproteins from tomato fruit pericarp were bound to three different mannose-binding lectins (Concanavalin A, ConA; Snowdrop lectin, GNA; and Lentil lectin, LCH). After trypsin digestion N-glycopeptides were fractionated using graphitic carbon (PGC) chromatography. Peptides bound to the PGC column, or present in the flow through, were analyzed by shotgun ESI-MS sequencing. Peptides bound to PGC were further fractionated by hydrophilic interaction chromatography (HILIC). Five main fractions were collected and divided into two aliquots for subsequent identification of N-glycosylation sites and analysis of intact N-glycopeptides. After bioinformatics analysis, a low abundant glycoprotein (XEGIP) was selected for recombinant expression in planta followed by purification and MS analysis to determine the N-glycan structures.
