Abstract
Background/Aims
This study investigated the role of single nucleotide polymorphisms (SNPs) near the interleukin-28B (IL28B) gene with respect to clinical outcomes and the antiviral response in hepatitis C virus (HCV) infection to suggest the practical utility of IL28B genotyping in Korea.
Methods
Two SNPs near IL28B, rs12979860 and rs8099917, were analyzed using an allelic discrimination assay in a total of 454 individuals, including 147 health-check examinees and 307 patients with HCV infection.
Results
The CC genotype frequency was significantly higher in the spontaneous recovery group than in the chronic infection group and was higher in the chronic hepatitis group than in the liver cirrhosis or hepatocellular carcinoma group, suggesting its favorable role in the clinical outcome. Multivariate analysis revealed that the rs12979860 CC genotype was an independent predictor of sustained virologic response (SVR) in genotype 1 HCV infection. During the currently used response-guided therapy, IL28B genotyping was most helpful for the patients who exhibit early virologic responses without rapid virologic responses, as those patients exhibiting the non-CC type did not achieve SVR, although they represented approximately one-third of the total patients.
Conclusions
The IL28B SNP is an independent predictor of SVR. Our results may be helpful if the findings are carefully applied to select patients in Korea.
Keywords: Hepatitis C, Interleukin-28B, Recovery, Treatment, Korea
INTRODUCTION
About 130 to 170 million people were infected with hepatitis C virus (HCV) worldwide.1 Globally, 27% of liver cirrhosis (LC) and 25% of hepatocellular carcinoma (HCC) were attributable to HCV.2 About 50% to 80% of patients with HCV infection progress to chronic infection which can lead to LC or HCC.3 Standard treatment for hepatitis C infection is a combination therapy with pegylated-interferon α and ribavirin but currently new standard care was suggested with triple combination therapy including direct antiviral agents (i.e., boceprevir or telaprevir) aiming to achieve sustained virologic response (SVR).4,5 Interestingly, compared to Caucasian patients, Asian patients showed higher rate of spontaneous clearance of HCV in acute hepatitis C,6 and higher SVR rate after antiviral therapy in chronic hepatitis C,7 although the mechanism of this phenomenon was unclear.
Recently, several genome wide association studies (GWAS) showed that single nucleotide polymorphism (SNP) near interleukin-28B (IL28B) gene is associated with SVR rate in HCV genotype 1 infection regardless of different ethnic group.8-11 Especially in the treatment naïve patients, IL28B genotype is related to the response in patients receiving triple therapy.12 Therefore, several centers adapt the genotyping of IL28B SNP to predict SVR before antiviral therapy and to minimize useless treatment. Although one of the GWAS was reported in Japan, there were not enough studies on the role of IL28B polymorphism in the clinical outcomes or SVR in the Asian patients including Koreans. The objectives of this study were to investigate the association between IL28B SNP and clinical outcomes of HCV infection, to evaluate the association between IL28B SNP and SVR in genotype 1 infection, and to suggest the practical utility of IL28B genotyping under the current strategy of response-guided therapy (RGT).
MATERIALS AND METHODS
1. Patients
Study subjects are comprised of the two groups: 147 health check examinees enrolled in Seoul National University Bundang Hospital, and 307 patients infected with HCV who were retrospectively enrolled in three university hospitals from January 2007 to December 2010. The health check examinees were selected after matching age and sex distribution to the standard Korean population according to the census for Korean population in 2007, so that they were a surrogate group of general population. Among the 307 patients with HCV infection, 47 patients were spontaneously recovered from acute infection without antiviral therapy showing a positive result for anti-HCV and repeatedly negative results for HCV RNA at least two times with normal liver function tests (spontaneous recovery group). The chronic infection group included 260 patients showing a positive serum HCV RNA for more than 6 months, which were classified into chronic hepatitis group (174 patients), LC group (53 patients), and HCC group (33 patients). LC was diagnosed according to the compatible biopsy findings, or clinical findings such as varices, ascites, and compatible radiologic findings. HCC was diagnosed according to the criteria suggested in American Association for the Study of Liver Diseases guideline.13 Exclusion criteria were as follows: 1) age less than 18 years; 2) coinfection with chronic hepatitis B or human immunodeficiency virus.
In the chronic infection group, 151 patients (58%) were infected by genotype 1 HCV (mostly genotype 1b) and 109 patients (42%) by genotype 2 (mostly genotype 2a). In the genotype 1 HCV-infected 151 patients, 70 patients were treated and evaluable on the achievement of SVR. Among the 70 patients, 55 patients had complete results on the rapid virologic response (RVR), early virologic response (EVR), and SVR.
2. Response-guided standard antiviral therapy
Pegylated interferon α-2a (180 µg, Pegasys®; Roche, Basel, Switzerland) or pegylated interferon α-2b (1.5 µg/kg, Pegintron®; MSD, Whitehouse Station, NJ, USA) was injected subcutaneously once a week and ribavirin (Viramid®; Ilsung Pharmaceuticals Co., Seoul, Korea) was administered orally twice daily at a dose of 1,000 to 1,200 mg/day for 48 weeks in genotype 1 patients; 800 mg/day for 24 weeks in genotype 2 patients. RVR was defined as a negative serum HCV RNA at 4 weeks of treatment. EVR was defined as ≥2 log10 reduction in serum HCV RNA level at 12 weeks of therapy, and SVR was defined as undetectable serum HCV RNA at 6 months after completion of therapy.
3. Laboratory evaluation
All patients tested positive for serum anti-HCV antibodies (ADVIA centaur® XP assay; Bayer Healthcare LLC, Tarrytown, NY, USA). The HCV RNA was amplified using RNA polymerase chain reaction (PCR) and hybridization methods (lower limit of detection 50 IU/mL, COBAS® Amplicor HCV test version 2.0; Roche Molecular Systems, Branchburg, NJ, USA), and the serum concentration of HCV RNA was measured using real-time PCR (COBAS® AmpliPrep/COBAS® TaqMan® HCV Test; Roche Molecular Systems).
4. Genotypic analysis of IL28B SNP
Genomic DNA was isolated from peripheral blood leukocytes using the DNA purification kit. Two SNPs near IL28B, rs12979860 and rs8099917, were analyzed using a TaqMan 5' allelic discrimination assay. PCR reactions were carried out in a total volume of 25 µL with the following amplification protocol: preincubation at 60℃ for 1 minute and at 95℃ for 10 minutes, followed by 40 cycles of 95℃ for 15 seconds and 60℃ for 1 minute. The PCR results and genotype of each sample were automatically attributed by the SDS 2.2.1 software for allelic discrimination (Applied Biosystems, Foster City, CA, USA).
5. Statistical analysis
The differences in numeric variables and categorical variables were tested with Student t-test and with chi-square tests or Fisher exact tests as appropriate. The multivariate logistic regression analysis was planned using variables showing p-value of less than 0.10 in the univariate analysis. Statistical analysis was conducted using PASW statistics 18.0 (SPSS Inc., Chicago, IL, USA), and p-value of less than 0.05 was considered to be significant.
6. Ethics statement
This study was approved by Institutional Review Board of Seoul National University Bundang Hospital, and the written informed consent was received from every participant.
RESULTS
1. Clinical characteristics and genotypic frequency of IL28B polymorphism
The clinical characteristics of the health check examinees and the HCV infected patients are summarized in Table 1. The rs12979860 allele frequency in the health check examinees was 88.4% for CC type, 10.9% for CT type and 0.7% for TT type. The genotypic distribution of rs8099917 was very similar to that of rs12979860 but not identical in this study. The estimated level of linkage disequilibrium from genotype frequency data showed that the two loci are tightly linked with r2 of 0.87 (data not shown).
Table 1.
The Clinical Characteristics and Genotype Frequency of Interleukin 28B (rs12979860 and rs8099917) in Health Check Examinees and Patients with Hepatitis C Viral Infection
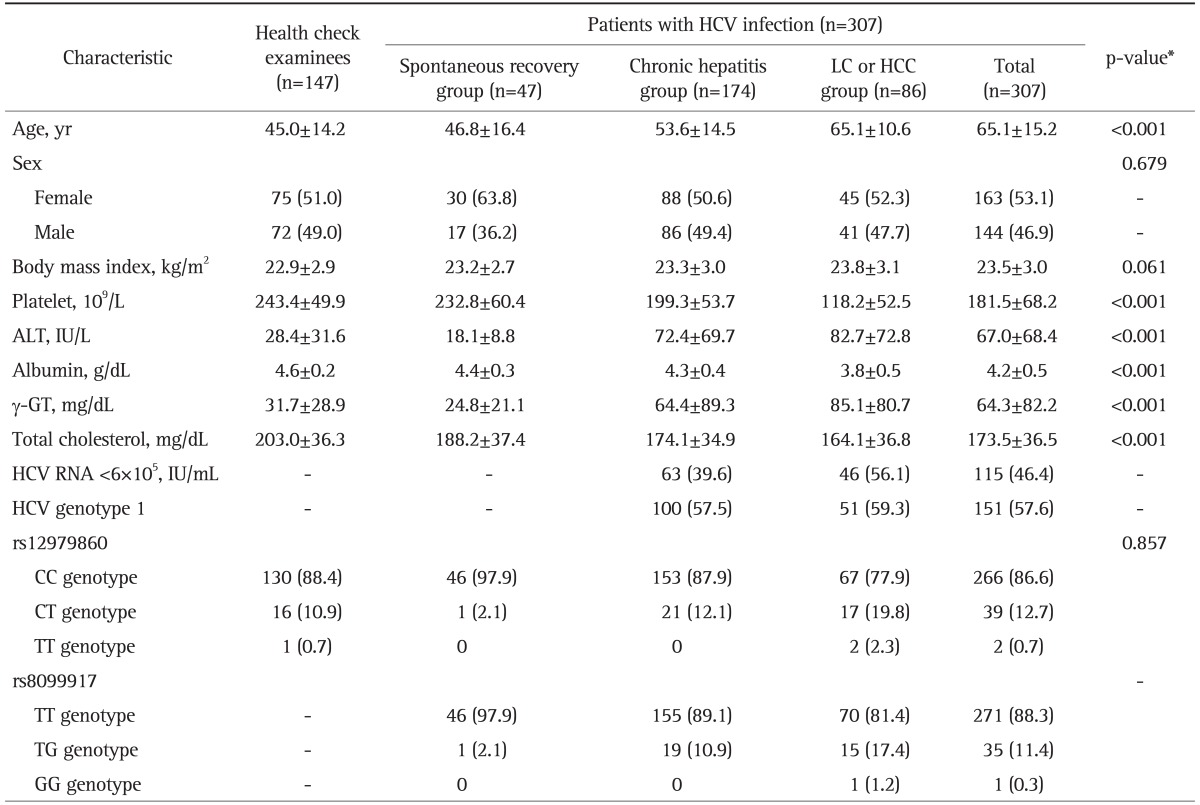
Values are presented as mean±SD or number (%).
HCV, hepatitis C virus; LC, liver cirrhosis; HCC, hepatocellular carcinoma; ALT, alanine aminotransferase.
*p-value comparing the health check examinees (n=147) and patients with HCV infection (n=307).
2. Association between IL28B polymorphism and clinical outcome of HCV infection
The frequency of rs12979860 CC type in the spontaneous clearance group (97.9%, n=47) was significantly higher than that (84.6%, n=260) in chronic infection group (p=0.014) (Table 1). In the chronic infection group, the CC genotype was significantly higher in chronic hepatitis group (87.9%, n=174) than in LC or HCC group (77.9%, n=86) (p=0.035). On the multivariate logistic regression analysis, rs12979860 CC type and young age less than 45 years were the independent factors for spontaneous clearance (odds ratio [OR], 8.29; 95% confidence interval [CI], 1.09 to 63.26) (Table 2) and for the better clinical outcome such as chronic hepatitis rather than LC or HCC (Table 3). It suggests the significant role of rs12979860 CC type in a favorable clinical outcome of HCV infection.
Table 2.
Results of Univariate and Multivariate Analyses of the Factors Associated with Spontaneous Clearance of the Hepatitis C Virus (HCV) in HCV-Infected Patients
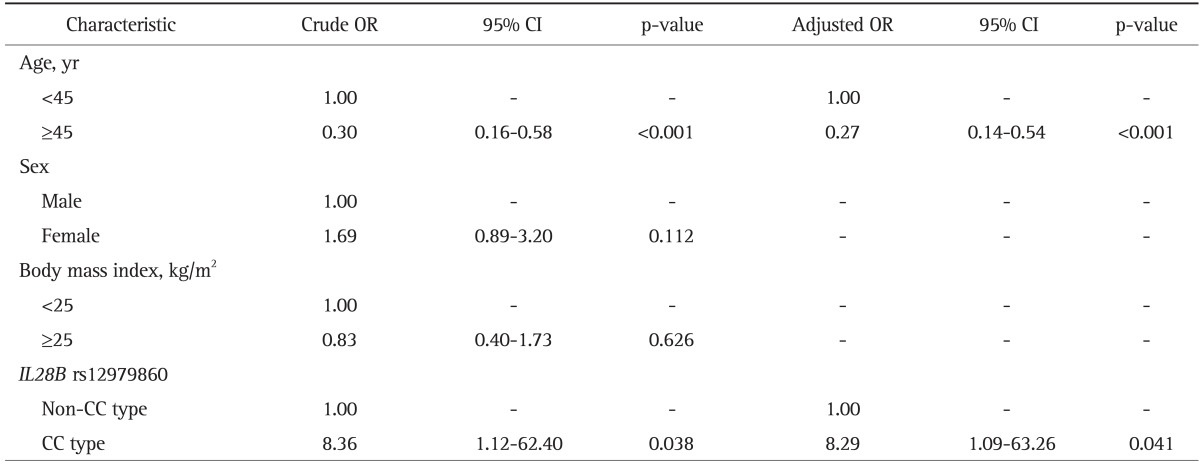
OR, odds ratio; CI, confidence interval; IL28B, interleukin-28B.
Table 3.
Results of Univariate and Multivariate Analyses of the Factors Associated with Advanced Liver Disease (Liver Cirrhosis or Hepatocellular Carcinoma) in Patients with Chronic Hepatitis C Viral Infection
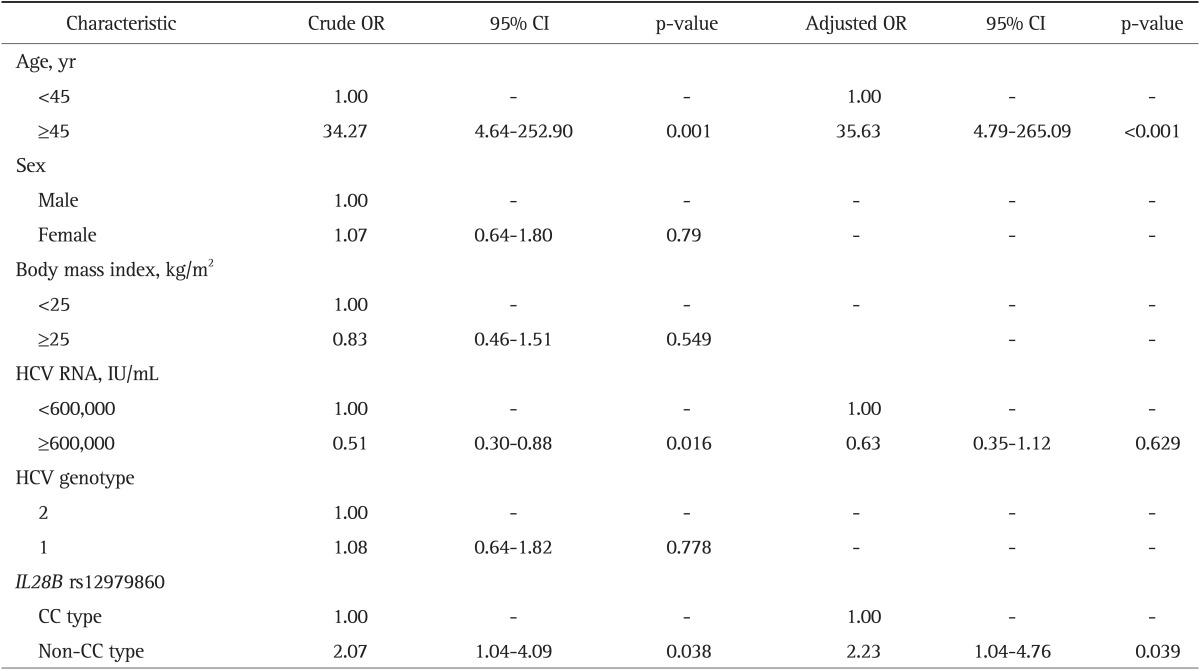
OR, odds ratio; CI, confidence interval; HCV, hepatitis C virus; IL28B, interleukin-28B.
3. Association between IL28B polymorphism and SVR in genotype 1 HCV infection
In a total of 260 patients of chronic infection group, 151 patients (58%) were infected by genotype 1 HCV and 109 patients (42%) by genotype 2. In the 151 patients with genotype 1 infection, 70 patients were treated and evaluable on the achievement of SVR. In the 70 patients with genotype 1 infection, SVR rate was 60%, and the frequency of rs12979860 CC type in SVR group (n=42) was 97.6%, while in non-SVR group (n=28) was 64.3%.
On multivariate logistic regression analysis, rs12979860 CC genotype was an independent factor for SVR in the genotype 1 HCV infection (OR, 21.09; 95% CI, 2.37 to 187.82), as well as platelet count ≥140,000/µL (Table 4). Moreover, rs12979860 CC genotype was an independent factor for RVR in the genotype 1 HCV infection (OR, 22.17; 95% CI, 2.23 to 220.84), as well as HCV RNA <600,000 IU/mL on multivariate logistic regression analysis (Table 5).
Table 4.
Results of Univariate and Multivariate Analyses of the Factors Associated with Sustained Virologic Response in Patients with Hepatitis C Virus Genotype 1 Infection
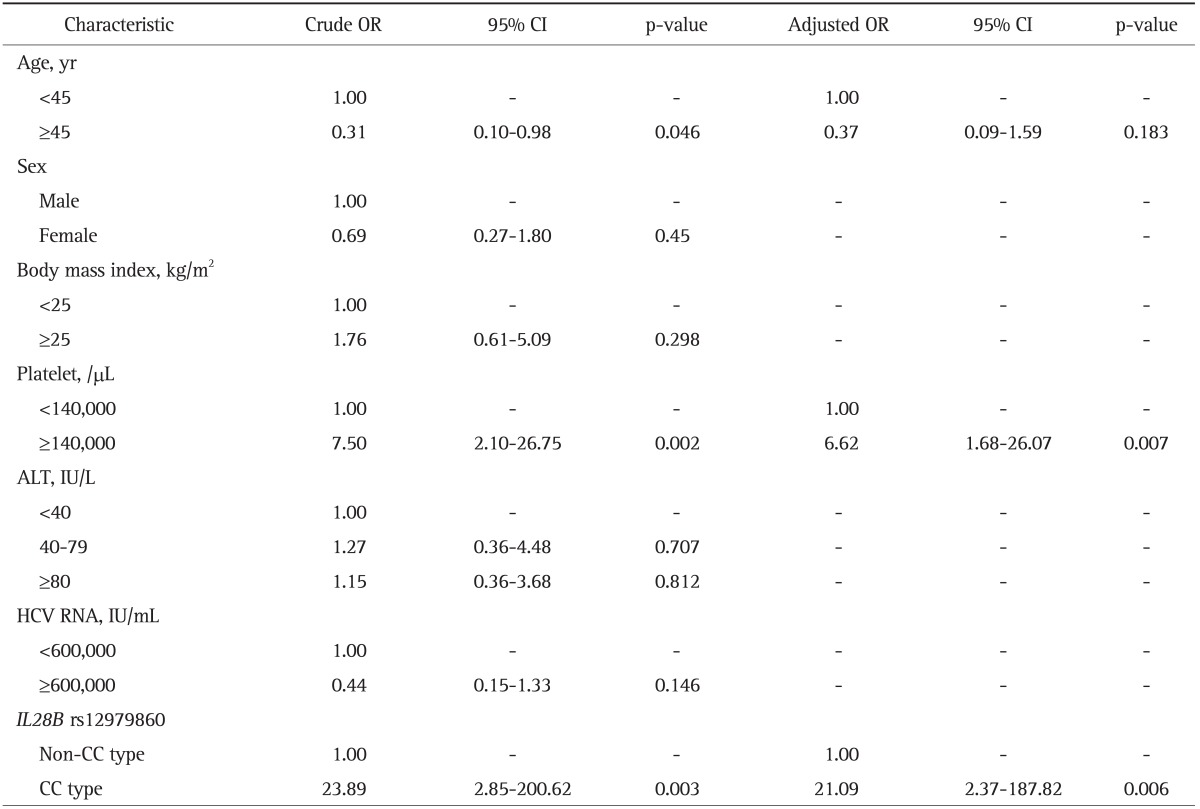
OR, odds ratio; CI, confidence interval; ALT, alanine aminotransferase; HCV, hepatitis C virus; IL28B, interleukin-28B.
Table 5.
Results of Univariate and Multivariate Analyses of the Factors Associated with Rapid Virologic Response in Patients with Hepatitis C Virus Genotype 1 Infection
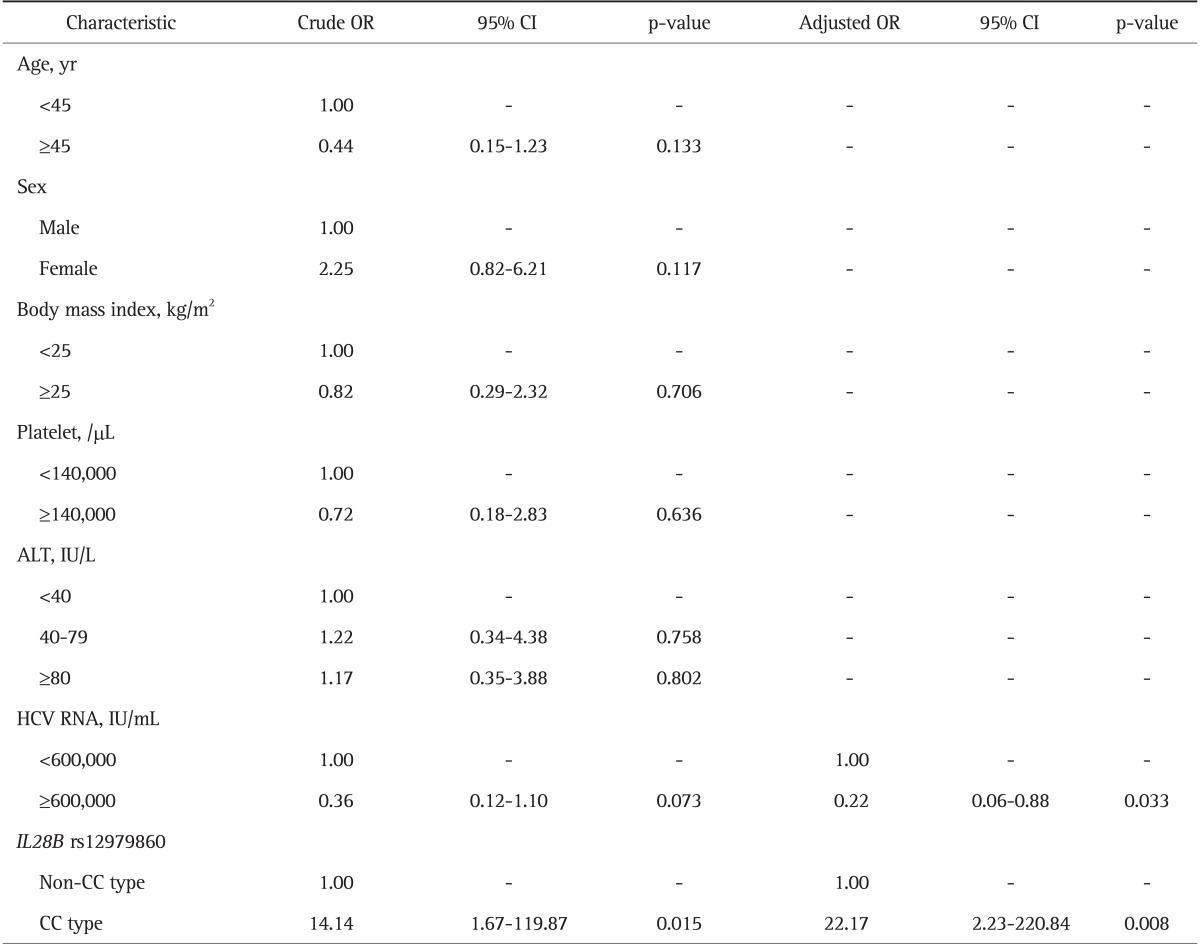
OR, odds ratio; CI, confidence interval; ALT, alanine aminotransferase; HCV, hepatitis C virus; IL28B, interleukin-28B.
On the other hand, in the 109 patients with genotype 2 infection, 58 patients were evaluable on SVR, which showed that SVR rate was 84.5%: the frequency of rs12979860 CC type in SVR group (n=49) was 87.8%, while in non-SVR group (n=9) was 88.9% (p=0.924). And rs12979860 CC genotype was not related to RVR in patients with genotype 2 HCV infection (p=0.769).
4. Role of IL28B SNP analysis in the current RGT
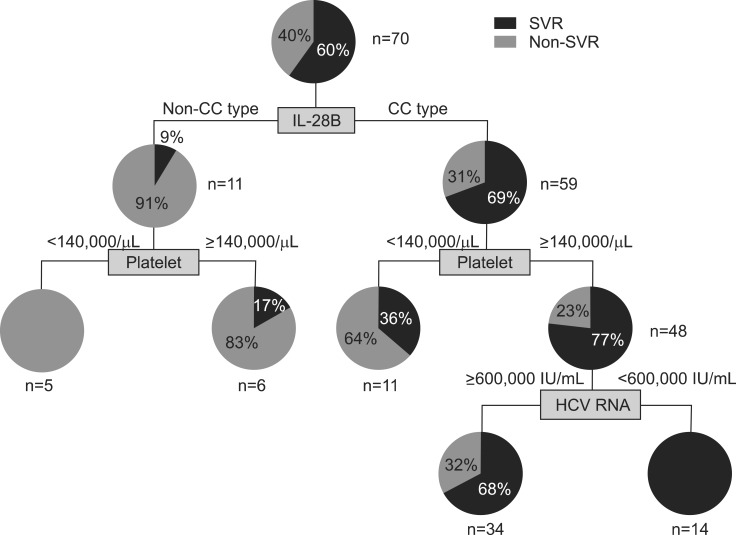
A suggested algorithm using IL28B genotype and platelet count was displayed in Fig. 1 for the 70 patients with HCV genotype 1 infection who were treated and evaluable on the achievement of SVR. Among the total 70 patients, five patients (7.1%) showing IL28B non-CC type and low platelet count lower than 140,000/µL did not achieve SVR, while 48 patients (68.6%) showing IL28B CC type and platelet count higher than 140,000/µL showed SVR rate of 77%. In between, SVR rates were estimated from 17% to 36%. If HCV RNA level was added in the algorithm, all of 14 patients having IL28B CC type, normal platelet count and low HCV RNA level achieved SVR. Therefore, this algorithm could give an expected probability of SVR for each patient before antiviral therapy.
Fig. 1.
A suggested algorithm for the expected probability of sustained virologic response (SVR) according to the presence of the interleukin-28B (IL28B) polymorphism (rs12979860), platelet count, and hepatitis C virus (HCV) RNA levels in Korean patients infected with HCV genotype 1. Among the 70 total patients included in this algorithm, all five patients exhibiting the IL28B non-CC type and a low platelet count (7%) did not achieve SVR. However, all 14 patients exhibiting the IL28B CC type, a normal platelet count, and low HCV RNA levels (20%) achieved SVR. Between these extremes, SVR rates ranged from 17% to 68%.
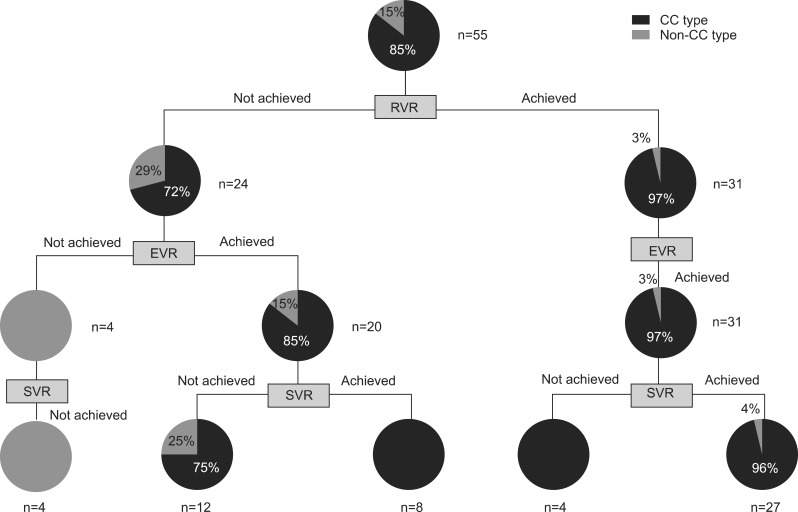
Among the 70 patients with genotype 1 infection, 55 patients had complete results on the RVR, EVR and SVR. Using those 55 patients, RGT algorithm after initiation of antiviral therapy was presented in Fig. 2. RVR was achieved in 31 patients (56.4%), all of them achieved EVR, and 30 patients (97%) showed IL28B CC type. In other hand, only 4 patients (7.3%) did not achieve EVR, and did not achieve SVR, either. Therefore, those with RVR (n=31) should continue the antiviral therapy, and those without EVR (n=4) should discontinue the antiviral therapy at 12 weeks of therapy, regardless of their IL28B genotypes. It suggests that at least a total 34 patients (64%) may not need to do their IL28B SNP genotyping for the therapeutic decision once they initiated the antiviral therapy. Therefore, IL28B SNP genotyping was most helpful to the patients who showed EVR without RVR (n=20, 36%), because those patients having non-CC type did not achieve SVR.
Fig. 2.
Practical value of the interleukin-28B (IL28B) polymorphism (rs12979860) under the current strategy of response-guided therapy in Korean patients infected with hepatitis C virus (HCV) genotype 1. According to the current response-guided treatment algorithm, all of the patients with rapid virologic response (RVR) (31 of 55, 56%) should continue the defined duration of therapy, and all of the patients without early virologic response (EVR) (4 of 55, 7%) should discontinue the antiviral therapy regardless of the result of the IL28B genotyping, which suggests that there is no need to determine the IL28B SNP genotype in 63% of the patients. IL28B single nucleotide polymorphism (SNP) genotyping may be helpful only in those patients achieving EVR without RVR (36%) because patients with the non-CC type did not achieve sustained virologic response (SVR).
DISCUSSION
In this study, in concordance with other reports,14,15 about 88% of Korean population had rs12979860 CC type of IL28B SNP, and it affected spontaneous clearance, benign clinical outcome of HCV infection, and SVR in genotype 1 HCV infection. The genotypic distribution of rs8099917 was very similar to that of rs12979860, but not identical. The algorithm using IL28B genotype and platelet count presents an expected probability of SVR in genotype 1 HCV infected patients before antiviral therapy. However, once the therapy is initiated, practical utility of IL28B genotyping seems to be limited under current strategy of RGT.
The genotypic frequency of rs12979860 in the health check examinee of this study showed that CC type in 88.4%, CT type 10.9%, and TT type in 0.7%: C allele frequency of 93.9%, and T allele frequency of only 6.1%. Thomas et al.16 reported the rs12979860 C allele frequency in worldwide population, in that C allele frequency was 23% to 55% in African populations, 52% to 80% in European population, 75% to 98% in Southwest Asians, and 90% to 100% in East Asian populations including Koreans (93.5%, n=54), Chinese (93.6%, n=47), and Japanese (91.0%, n=50), which was well compatible to our results. The genotypic distribution of rs8099917 was similar to that of rs12979860, because of the strong linkage disequilibrium of the two SNPs.
In this study, rs12979860 CC genotype was strongly associated with spontaneous clearance of HCV infection (OR, 8.29; 95% CI, 1.09 to 63.26; p=0.041), which was compatible to the other reports.16,17 Grebely et al.18 suggested an algorithm for clinical management of acute HCV infection using IL28B genotyping; if IL28B genotype of patients with recent HCV infection was rs8099917 GG or GT type, antiviral therapy was promptly suggested without waiting for spontaneous clearance. After acute HCV infection, about 50% to 90% of patients experience chronic infection according to the presence of multiple cofactors4,19 as well as the IL28B genotype. In addition, more advanced complication of HCV infection such as LC or HCC was also significantly related to non-CC type of IL28B in this study. The multiple logistic regression analysis confirmed that IL28B non-CC type was independently associated with LC or HCC (OR, 2.23; 95% CI, 1.04 to 4.76; p=0.039). Fabris et al.20 showed that rs12979860 T allele was more prevalent in patients with HCV-related cirrhosis than in other etiologies of cirrhosis, and carriage of this allele seemed to augment the risk of developing HCC in Italian patients with HCV-related cirrhosis who underwent liver transplantation. These results suggest that IL28B genotype shows a strong effect on the clinical outcomes of HCV infection. However, because of the small sample size in this study, further study to confirm the result is warranted.
In concordance with previous GWAS results,8-11 this study also confirmed that rs12979860 CC genotype was strongly associated with SVR in Korean patients with HCV genotype 1 infection (OR, 21.09; 95% CI, 2.37 to 182.82; p=0.006), as well as platelet count, which was an indirect marker of advanced fibrosis. This result suggests that high frequency of IL28B CC type in Asians including Koreans can be an explanation for a higher SVR reported in Asians than in Caucasians or African Americans. However, the IL28B genotype was not associated with SVR in genotype 2 infection in this study, which was controversial.11,14,21-23
The molecular mechanism for IL28B in the pathogenesis of HCV infection was not elucidated. Nevertheless, IL28B seems to play an important role in immune response. The inhibition of HCV replication in vitro by interferon-λ showed the similar efficiency to type 1 interferon, and clinical trials using pegylated interferon-λ in human patients showed similar antiviral effect to pegylated interferon-α.24,25 Recent experimental study showed anti-tumor activity for interferon-λ in various cancer cell lines.26,27
As reported in the previous study,28 we presented a pretreatment prediction algorithm for SVR using IL28B genotype and platelet count in 70 patients with genotype 1 HCV infection, which provides a good prediction model for SVR before antiviral therapy. The IL28B SNP is the most useful predictor before antiviral therapy, and the RVR is that during antiviral therapy. To avoid some adverse events developed during the first 4 weeks of antiviral therapy, IL28B genotyping should be performed. If then, should we perform IL28B genotyping for all the patients who are going to receive antiviral therapy in the real practice setting? According to the suggested algorithm, once the therapy is initiated, practical utility of IL28B genotyping seems to be limited under current strategy of RGT, because about 63% of the treated patients including the patients with RVR and the patients without EVR may not need to do their IL28B SNP genotyping for the therapeutic decision. As long as we follow RGT for East Asian patients, routine testing of the IL28B SNP in HCV genotype 1 infection should be considered in the context of adequate cost-effectiveness, because almost all of them have IL28B CC genotype, and RGT itself determines about two-thirds of the response. In the meanwhile, IL28B SNP genotyping might be helpful for the patients who showed EVR but no RVR. However, this result should be validated in the further study, because of small number of included patients. There are several limitations of this study such as the retrospective design and incomplete data on the RVR or EVR, especially lack of data on complete EVR, the small sample size and the cross-sectional nature of the study.
In conclusion, about 90% of Koreans had the rs12979860 CC genotype of IL28B SNP, and the IL28B SNP was an independent predictor of spontaneous clearance, favorable clinical outcomes of HCV infection, and SVR in genotype 1 HCV infection. It may be helpful in the clinical settings if the findings were carefully applied to selected patients in Korea.
ACKNOWLEDGEMENTS
This study was supported by research grant from the Korea National Institute of Health, Grant no. 2011-E4-200300.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29(Suppl 1):74–81. doi: 10.1111/j.1478-3231.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 2.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245–264. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 5.Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB American Association for Study of Liver Diseases. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:1433–1444. doi: 10.1002/hep.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JY, Won JE, Jeong SH, et al. Acute hepatitis C in Korea: different modes of infection, high rate of spontaneous recovery, and low rate of seroconversion. J Med Virol. 2011;83:1195–1202. doi: 10.1002/jmv.22100. [DOI] [PubMed] [Google Scholar]
- 7.Yu ML, Chuang WL. Treatment of chronic hepatitis C in Asia: when East meets West. J Gastroenterol Hepatol. 2009;24:336–345. doi: 10.1111/j.1440-1746.2009.05789.x. [DOI] [PubMed] [Google Scholar]
- 8.Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka Y, Nishida N, Sugiyama M, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105–1109. doi: 10.1038/ng.449. [DOI] [PubMed] [Google Scholar]
- 10.Suppiah V, Moldovan M, Ahlenstiel G, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100–1104. doi: 10.1038/ng.447. [DOI] [PubMed] [Google Scholar]
- 11.Rauch A, Kutalik Z, Descombes P, et al. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: a genome-wide association study. Gastroenterology. 2010;138:1338–1345. doi: 10.1053/j.gastro.2009.12.056. [DOI] [PubMed] [Google Scholar]
- 12.Jensen DM, Pol S. IL28B genetic polymorphism testing in the era of direct acting antivirals therapy for chronic hepatitis C: ten years too late? Liver Int. 2012;32(Suppl 1):74–78. doi: 10.1111/j.1478-3231.2011.02712.x. [DOI] [PubMed] [Google Scholar]
- 13.Bruix J, Sherman M American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinn DH, Kim YJ, Lee ST, et al. Association of a single nucleotide polymorphism near the interleukin-28B gene with response to hepatitis C therapy in Asian patients. J Gastroenterol Hepatol. 2011;26:1374–1379. doi: 10.1111/j.1440-1746.2011.06744.x. [DOI] [PubMed] [Google Scholar]
- 15.Lyoo K, Song MJ, Hur W, et al. Polymorphism near the IL28B gene in Korean hepatitis C virus-infected patients treated with peg-interferon plus ribavirin. J Clin Virol. 2011;52:363–366. doi: 10.1016/j.jcv.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tillmann HL, Thompson AJ, Patel K, et al. A polymorphism near IL28B is associated with spontaneous clearance of acute hepatitis C virus and jaundice. Gastroenterology. 2010;139:1586–1592. doi: 10.1053/j.gastro.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Grebely J, Petoumenos K, Hellard M, et al. Potential role for interleukin-28B genotype in treatment decision-making in recent hepatitis C virus infection. Hepatology. 2010;52:1216–1224. doi: 10.1002/hep.23850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santantonio T, Wiegand J, Gerlach JT. Acute hepatitis C: current status and remaining challenges. J Hepatol. 2008;49:625–633. doi: 10.1016/j.jhep.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Fabris C, Falleti E, Cussigh A, et al. IL-28B rs12979860 C/T allele distribution in patients with liver cirrhosis: role in the course of chronic viral hepatitis and the development of HCC. J Hepatol. 2011;54:716–722. doi: 10.1016/j.jhep.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 21.McCarthy JJ, Li JH, Thompson A, et al. Replicated association between an IL28B gene variant and a sustained response to pegylated interferon and ribavirin. Gastroenterology. 2010;138:2307–2314. doi: 10.1053/j.gastro.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawaoka T, Hayes CN, Ohishi W, et al. Predictive value of the IL28B polymorphism on the effect of interferon therapy in chronic hepatitis C patients with genotypes 2a and 2b. J Hepatol. 2011;54:408–414. doi: 10.1016/j.jhep.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 23.Yu ML, Huang CF, Huang JF, et al. Role of interleukin-28B polymorphisms in the treatment of hepatitis C virus genotype 2 infection in Asian patients. Hepatology. 2011;53:7–13. doi: 10.1002/hep.23976. [DOI] [PubMed] [Google Scholar]
- 24.Shiffman M, Lawitz E, Zaman A, et al. PEG-IFN-l: antiviral activity and safety profile in a 4-week phase 1b study in relapsed genotype 1 hepatitis C infection. J Hepatol. 2009;50(Suppl 1):S237. [Google Scholar]
- 25.Zeuzem S, Arora S, Bacon B, et al. Pegylated interferon-lambda (PEGIFN-λ) shows superior viral response with improved safety and tolerability versus PEGIFNα-2a in HCV patients (Gl/2/3/4): emerge phase IIB through week 12. J Hepatol. 2011;54(Suppl 1):S538–S539. [Google Scholar]
- 26.Abushahba W, Balan M, Castaneda I, et al. Antitumor activity of type I and type III interferons in BNL hepatoma model. Cancer Immunol Immunother. 2010;59:1059–1071. doi: 10.1007/s00262-010-0831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q, Kawamura K, Ma G, et al. Interferon-lambda induces G1 phase arrest or apoptosis in oesophageal carcinoma cells and produces anti-tumour effects in combination with anti-cancer agents. Eur J Cancer. 2010;46:180–190. doi: 10.1016/j.ejca.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Kurosaki M, Tanaka Y, Nishida N, et al. Pre-treatment prediction of response to pegylated-interferon plus ribavirin for chronic hepatitis C using genetic polymorphism in IL28B and viral factors. J Hepatol. 2011;54:439–448. doi: 10.1016/j.jhep.2010.07.037. [DOI] [PubMed] [Google Scholar]