FIGURE 2.
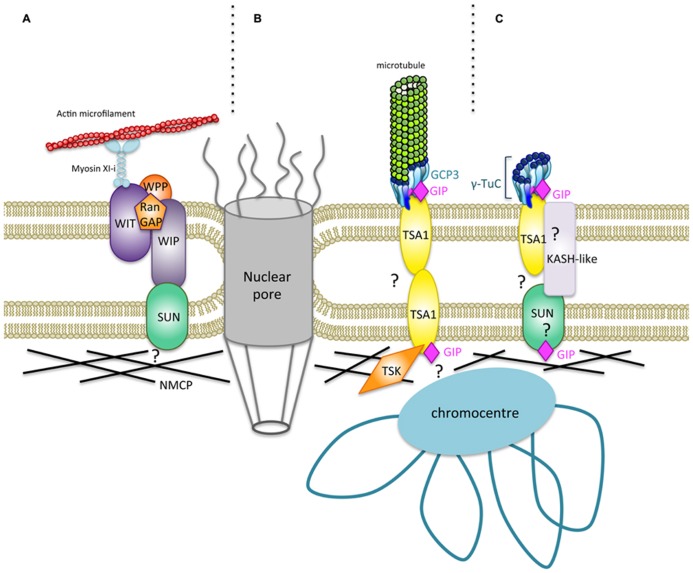
Hypothetical model for a new type of nucleocytoplasmic bridges regulating the shape of Arabidopsis thaliana nuclei. (A) A specific plant LINC complex has been characterized, and it plays a role in nuclear movement and shaping (Meier and Brkljacic, 2009; Graumann and Evans, 2010; Zhou et al., 2012; Tamura et al., 2013). Trimeric organization of SUN domain proteins (Sosa et al., 2012) has been omitted for more clarity. A WIT–WIP complex interacts with WWP proteins and RanGAP. WIT also interacts with Myosin XI-i which links the actin cytoskeleton to the ONM. The WIP–SUN complex constitutes the first core LINC identified in plants. NMCPs/LINCs are plamina components (Ciska and Moreno Diaz de la Espina, 2013) which may interact with SUN (Graumann et al., 2013). (B) A new nucleocytoplasmic continuum may consist of GIPs which bind both GCP3, a component of the γ-TuC involved in MT nucleation at the NE, and TSA1, a putative transmembrane protein. TSK localizes in the nucleoplasm (Suzuki et al., 2004; Takeda et al., 2004; Ohno et al., 2011) and may associate with TSA1 at the INM. Both TSK and GIP binding domains of TSA1 are overlapping at its C-terminus. TSA1 possesses coiled-coil domains which may allow multimerization of the protein. Multimerization propensity of GIP/MZT1 proteins have also been reported and could reinforce the interaction with their partners (Batzenschlager et al., 2013; Dhani et al., 2013). Such a model may at least exist in cycling cells. (C) The continuum may also involve a subpopulation of γ-TuCs which does not nucleate MTs in differentiated cells. The GIP–TSA1 complex may interact with a still unknown KASH-like protein associated to SUN in the perinuclear space. The molecular interplay at the INM, plamina and chromatin interface needs to be further characterized.