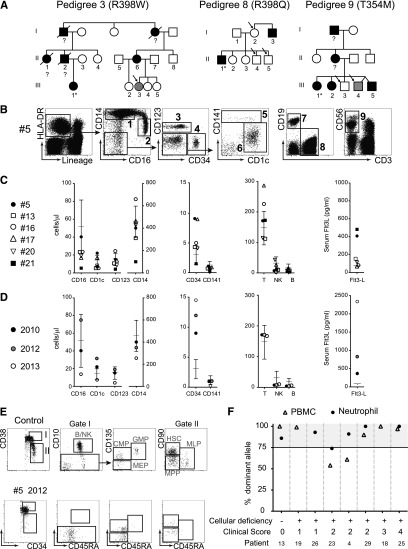
Figure 3.
Asymptomatic carriers of GATA2 mutation may develop cellular deficiency, elevated Flt3L, loss of BM progenitors, and clonal myelopoeisis. (A) Three pedigrees identified (mutation indicated) containing asymptomatic relatives (clinical core = 0), carrying GATA2 mutation (open symbols, arrowed). Gray symbols identify 2 patients with either elevated Flt3L (>200 pg/ml) or cytopenia. Filled symbols indicate affected patients with mutation (clinical score = 1 to 4). (B) DC, monocyte, and lymphocyte profiles of patient #5, 1 of 3 healthy carriers of GATA2 mutation showing a normal cellular phenotype at the first point of analysis in 2010. Populations: (1) CD14+ monocyte; (2) CD16+ monocyte; (3) pDC; (4) CD34+ progenitors; (5) CD141+ mDC; (6) CD1c+ mDC; (7) B cell; (8) T cell; and (9) NK cell. (C) Summary of DC and monocyte counts relative to reference ranges for the asymptomatic carriers. Case #5 (filled circle) is shown at first analysis in 2010. Case #21 (filled square) already has cytopenia. (D) Detailed analysis of case #5 showing the loss of cells and rising Flt3L over a 3-year period. (E) BM analysis of case #5 showing loss of B, NK, MLP, and GMP progenitors at midpoint when no cytopenia was evident. CMP, common myeloid progenitor; MEP, megakaryocyte-erythroid progenitor; MPP, multi-potent progenitor. (F) Pattern of X inactivation in females with GATA2 mutation at different stages of clinical evolution. Dominance of >75% is considered evidence of clonal hematopoiesis.