Table 2.
Nickel/iodide co-catalyzed epoxide ring opening with aryl halidesa
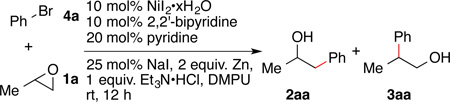 | |||
|---|---|---|---|
| entry | deviation from above | yield (%)b | 2:3 |
| 1 | none | 81 | > 95:5 |
| 2 | No TEA•HCl | 32 | 89:11 |
| 3 | 15 mol % NaI | 67 | > 95:5 |
| 4 | 25 mol % Bu4NI in place of NaI | 79 | 95:5 |
| 5 | 12.5 mol % ZnI2 in place of NaI | 46 | 88:12 |
| 6 | 12.5 mol % MnI2 in place of NaI | 61 | 95:5 |
| 7 | No NaI, 12 hours | 68 | > 95:5 |
| 8 | No NaI, 24 hours | 75 | > 95:5 |
| 9 | 25 mol % NaBr in place of NaI | 52 | > 95:5 |
| 12 | NiI2 in place of NiI2·xH2O | 86 | > 95:5 |
| 13 | 2 equiv. of Mn in place of Zn | 59 | 95:5 |
| 14c | 2 equiv. of TDAE in place of Zn | 48d | >95:5 |
| 15 | Heated to 60 °C for 12 h | 50 | 93:7 |
| 16 | I-Ph in place of Br-Ph | 51 | 91:9 |
Reactions were run with 1 equiv of Et3N•HCl and 2 equiv of zinc dust; 0.1 equiv nickel catalyst, ligand; 0.2 equiv pyridine, 0.25 equiv sodium iodide, and 1.34 equiv of 1a in 3 mL DMPU.
Uncorrected GC yield of 2aa.
TDAE = tetrakis(dimethylamino)ethylene.
Bromobenzene (52%) remained, but no biaryl or benzene was formed.
