Table 4.
Formation of branched products with Ni/Ti catalysisa
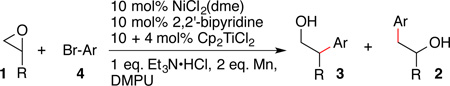 | ||||
|---|---|---|---|---|
| entry | R | Ar-Br | 3:2 | yield (%) |
| 1 | Me 1a | PhBr 4a | 3.3:1 | 70 (3aa) |
| 2 | C4H9 1b | 4a | 6:1 | 54 (3ab) |
| 3 | C6H13 1c | 4a | 99:1 | 41 (3ac) |
| 4 | 1a | p-MeO-C6H4Br 4b | 4:1 | 63 (3ba) |
| 5 | 1a | p-MeO2C-C6H4Br 4n | 3.5:1 | 62 (3na) |
Conditions: 2 equiv of Et3N•HCl and Mn dust were combined with 4 (1 equiv), 1 (1.34 equiv), and catalysts (10 mol%) in DMPU (0.17 M) and stirred for 12 h at rt. An additional portion of Cp2TiCl2was added after 2 h. Ratios of 3:2 were determined by GC analysis. Yield is an isolated mixture of 2 and 3.
