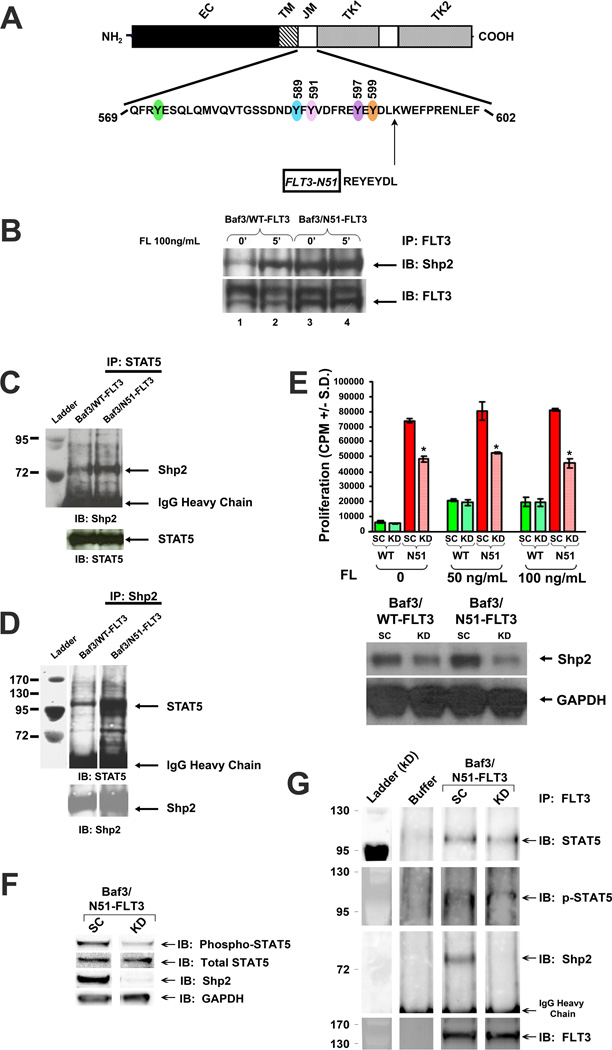
Figure 1. The protein tyrosine phosphatase, Shp2, interacts constitutively with FLT3 and STAT5 in FLT3-N51-expressing Baf3 cells and functionally contributes to FLT3-ITD-induced hyperproliferation.
(A) Schematic diagram showing amino acid duplication for the FLT3-N51 internal tandem duplication (ITD). (B) Total cellular protein extracts from baseline and FL-stimulated Baf3/WT-FLT3 or Baf3/N51-FLT3 cells were isolated, immunoprecipitated (IP) with anti-FLT3 and immunoblotted (IB) with anti-Shp2 and anti-FLT3. Total cellular protein extracts were immunoprecipitated (IP) with (C) anti-STAT5 or (D) anti-Shp2 and immunoblotted (IB) with anti-Shp2 or anti-STAT5, respectively. (E) 3H-thymidine incorporation and immunoblot analyses of Shp2 expression in Baf3/WT-FLT3 and Baf3/N51-FLT3 cells transfected with scrambled shRNA (SC) or shRNA specifically targeting Shp2 (KD). Representative of two independent experiments with similar results, n=4, *p<0.05 for N51 KD v. N51 SC, statistical analysis using unpaired, two-tailed, student’s t test. (F) Immunoblot analysis of Shp2 expression, STAT5 phosphorylation, and total STAT5 expression in Baf3/N51-FLT3 cells transfected with scrambled shRNA (SC) or shRNA specifically targeting Shp2 (KD). (G) Total cellular proteins from Baf3/N51-FLT3 cells transfected with scrambled shRNA (SC) or Shp2-specific shRNA (KD) were isolated, immunoprecipitated (IP) with anti-FLT3 and immunoblotted (IB) with anti-STAT5, anti-phospho-STAT5, anti-Shp2, and anti-FLT3.