Abstract
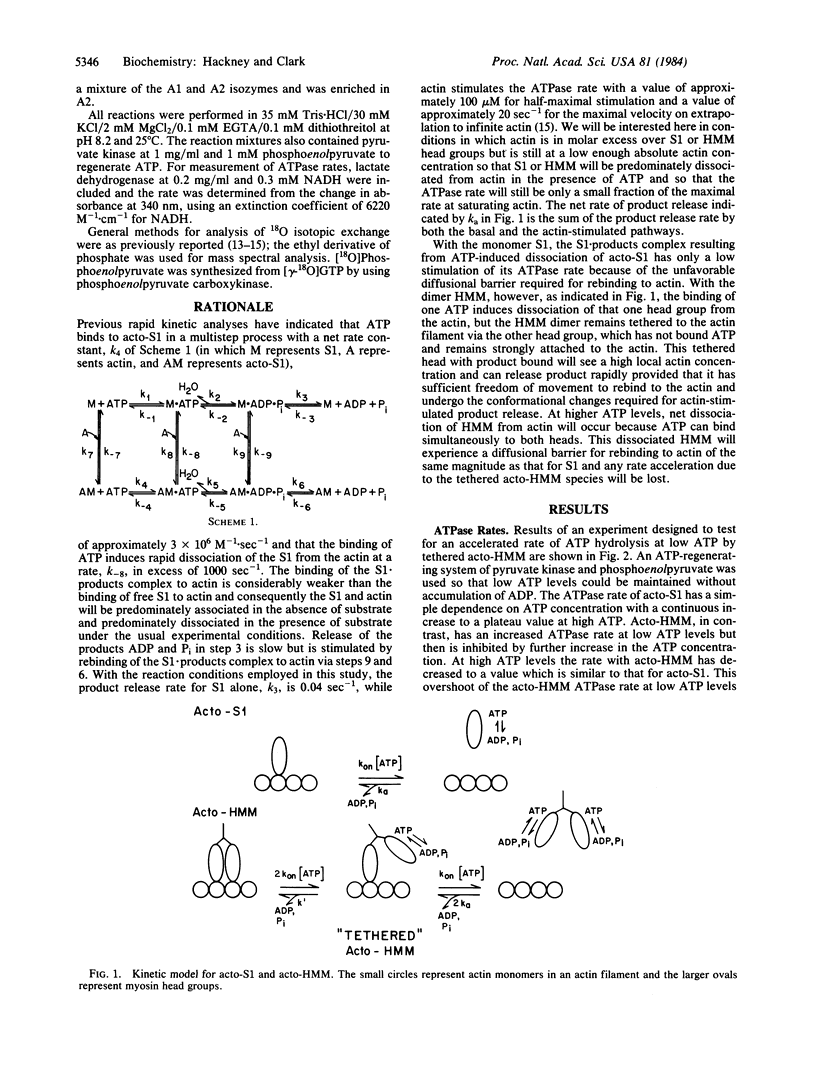
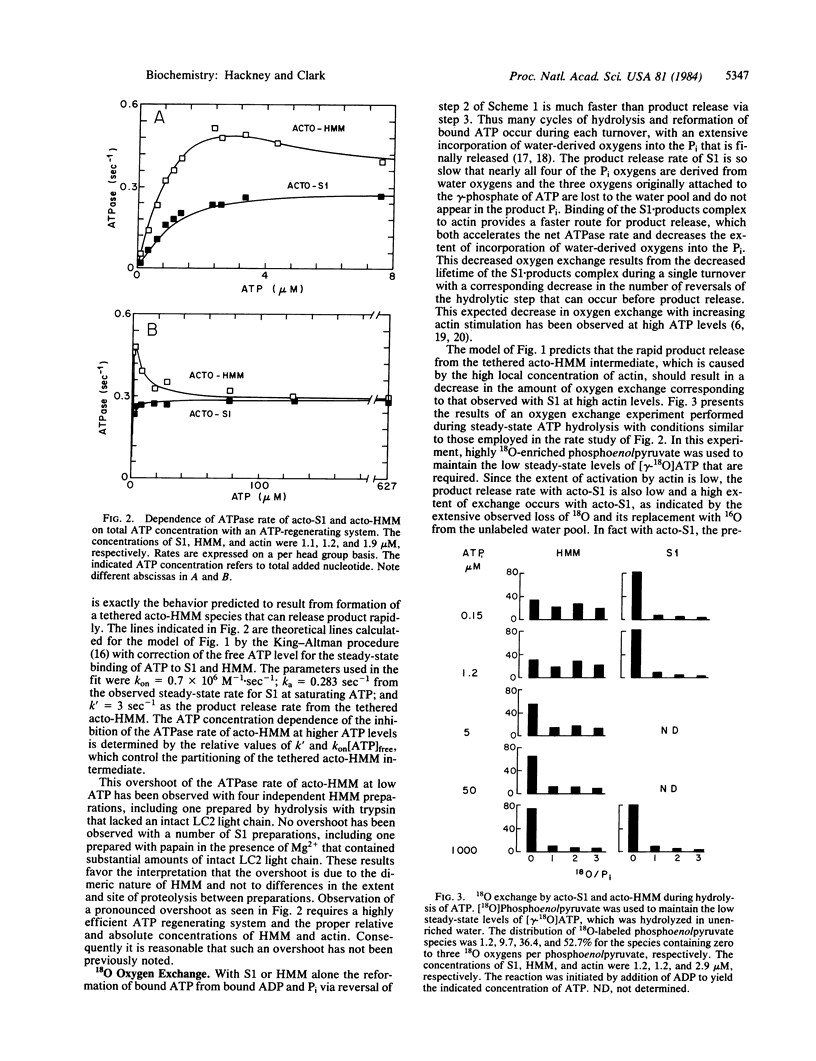
The influence of the supramolecular organization of myosin on its ATPase activity was investigated at a range of ATP concentrations, using as a model system subfragment 1 (S1) and heavy meromyosin (HMM), which are respectively monomeric and dimeric proteolytic fragments of myosin. At low ATP levels in the presence of a molar excess of actin, dimeric HMM showed an increased rate of ATP hydrolysis relative to that for monomeric S1. This increased ATPase for HMM was inhibited by high concentrations of ATP, which reduced the acto-HMM ATPase rate to the lower level of acto-S1. This observation is consistent with the rapid ATP hydrolysis of acto-HMM at low ATP being due to rapid product release from a "tethered" acto-HMM species, which has product bound to one head group while the other head group remains bound to actin. At high concentrations of ATP, ATP binds to both head groups, resulting in net dissociation of HMM from actin. This model is supported by 18O exchange data. Acto-HMM hydrolyzed ATP with extensive exchange of water oxygens into Pi at high ATP levels, but not at low ATP levels. Acto-S1 exhibited extensive exchange at both high and low ATP levels. This result is consistent with rapid product release from a tethered acto-HMM intermediate at low ATP.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Eisenberg E. Regulation and kinetics of the actin-myosin-ATP interaction. Annu Rev Biochem. 1980;49:921–956. doi: 10.1146/annurev.bi.49.070180.004421. [DOI] [PubMed] [Google Scholar]
- Bagshaw C. R., Trentham D. R., Wolcott R. G., Boyer P. D. Oxygen exchange in the gamma-phosphoryl group of protein-bound ATP during Mg2+-dependent adenosine triphosphatase activity of myosin. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2592–2596. doi: 10.1073/pnas.72.7.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantler P. D., Sellers J. R., Szent-Györgyi A. G. Cooperativity in scallop myosin. Biochemistry. 1981 Jan 6;20(1):210–216. doi: 10.1021/bi00504a035. [DOI] [PubMed] [Google Scholar]
- Greene L. E. Comparison of the binding of heavy meromyosin and myosin subfragment 1 in F-actin. Biochemistry. 1981 Apr 14;20(8):2120–2126. doi: 10.1021/bi00511a008. [DOI] [PubMed] [Google Scholar]
- Hackney D. D. Nonlinear dependence on actin of acto-heavy meromyosin 18O exchange and ATPase rate at low actin concentrations. J Biol Chem. 1982 Aug 25;257(16):9494–9500. [PubMed] [Google Scholar]
- Hackney D. D., Stempel K. E., Boyer P. D. Oxygen-18 probes of enzymic reactions of phosphate compounds. Methods Enzymol. 1980;64:60–83. doi: 10.1016/s0076-6879(80)64005-1. [DOI] [PubMed] [Google Scholar]
- Hackney D. D. Theoretical analysis of distribution of [18O]Pi species during exchange with water. Application to exchanges catalyzed by yeast inorganic pyrophosphatase. J Biol Chem. 1980 Jun 10;255(11):5320–5328. [PubMed] [Google Scholar]
- Highsmith S. Heavy meromyosin binds actin with negative cooperativity. Biochemistry. 1978 Jan 10;17(1):22–26. doi: 10.1021/bi00594a004. [DOI] [PubMed] [Google Scholar]
- Lymn R. W. Kinetic analysis of myosin and actomyosin atpase. Annu Rev Biophys Bioeng. 1979;8:145–163. doi: 10.1146/annurev.bb.08.060179.001045. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol. 1982;85(Pt B):55–71. doi: 10.1016/0076-6879(82)85009-x. [DOI] [PubMed] [Google Scholar]
- Mendelson R. A., Morales M. F., Botts J. Segmental flexibility of the S-1 moiety of myosin. Biochemistry. 1973 Jun 5;12(12):2250–2255. doi: 10.1021/bi00736a011. [DOI] [PubMed] [Google Scholar]
- Midelfort C. F. On the mechanism of actomyosin ATPase from fast muscle. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2067–2071. doi: 10.1073/pnas.78.4.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mornet D., Bertrand R., Pantel P., Audemard E., Kassab R. Structure of the actin-myosin interface. Nature. 1981 Jul 23;292(5821):301–306. doi: 10.1038/292301a0. [DOI] [PubMed] [Google Scholar]
- Persechini A., Hartshorne D. J. Phosphorylation of smooth muscle myosin: evidence for cooperativity between the myosin heads. Science. 1981 Sep 18;213(4514):1383–1385. doi: 10.1126/science.6455737. [DOI] [PubMed] [Google Scholar]
- Reisler E. Kinetic studies with synthetic myosin minifilaments show the equivalence of actomyosin and acto-HMM ATPases. J Biol Chem. 1980 Oct 25;255(20):9541–9544. [PubMed] [Google Scholar]
- Reisler E., Smith C., Seegan G. Myosin minifilaments. J Mol Biol. 1980 Oct 15;143(1):129–145. doi: 10.1016/0022-2836(80)90127-8. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Johnson K. A. Kinetic evidence for multiple dynein ATPase sites. J Biol Chem. 1983 Nov 25;258(22):13841–13846. [PubMed] [Google Scholar]
- Shukla K. K., Levy H. M. Mechanism of oxygen exchange in actin-activated hydrolysis of adenosine triphosphate by myosin subfragment 1. Biochemistry. 1977 Jan 11;16(1):132–136. doi: 10.1021/bi00620a022. [DOI] [PubMed] [Google Scholar]
- Shukla K. K., Levy H. M., Ramirez F., Marecek J. F., Meyerson S., Kuhn E. S. Distribution of [18O]Pi species from [gamma-18O]ATP hydrolysis by myosin and heavy meromyosin. Evidence for two kinds of myosin-active site differing in their rate of intermediate oxygen exchange. J Biol Chem. 1980 Dec 10;255(23):11344–11350. [PubMed] [Google Scholar]
- Sleep J. A., Boyer P. D. Effect of actin concentration on the intermediate oxygen exchange of myosin; relation to the refractory state and the mechanism of exchange. Biochemistry. 1978 Dec 12;17(25):5417–5422. doi: 10.1021/bi00618a015. [DOI] [PubMed] [Google Scholar]
- Sleep J. A., Hackney D. D., Boyer P. D. The equivalence of phosphate oxygens for exchange and the hydrolysis characteristics revealed by the distribution of [18O]Pi species formed by myosin and actomyosin ATPase. J Biol Chem. 1980 May 10;255(9):4094–4099. [PubMed] [Google Scholar]
- Sleep J. A., Hutton R. L. Actin mediated release of ATP from a myosin-ATP complex. Biochemistry. 1978 Dec 12;17(25):5423–5430. doi: 10.1021/bi00618a016. [DOI] [PubMed] [Google Scholar]
- Taylor E. W. Mechanism of actomyosin ATPase and the problem of muscle contraction. CRC Crit Rev Biochem. 1979;6(2):103–164. doi: 10.3109/10409237909102562. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Seidel J. C., Hyde J. S., Gergely J. Motion of subfragment-1 in myosin and its supramolecular complexes: saturation transfer electron paramagnetic resonance. Proc Natl Acad Sci U S A. 1975 May;72(5):1729–1733. doi: 10.1073/pnas.72.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. R., Eccleston J. F., Bagshaw C. R. Kinetic analysis of ATPase mechanisms. Q Rev Biophys. 1976 May;9(2):217–281. doi: 10.1017/s0033583500002419. [DOI] [PubMed] [Google Scholar]
- Webb M. R., Trentham D. R. The mechanism of ATP hydrolysis catalyzed by myosin and actomyosin, using rapid reaction techniques to study oxygen exchange. J Biol Chem. 1981 Nov 10;256(21):10910–10916. [PubMed] [Google Scholar]


