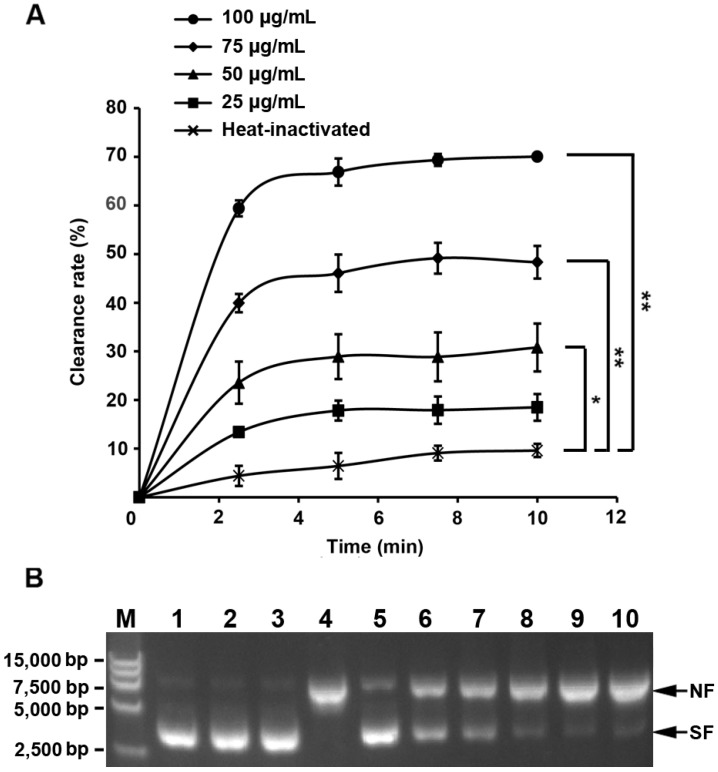
Figure 6.
Biological function of the recombinant CcPrx4 protein. (A) In vitro peroxidase activity of the CcPrx4 protein in the presence of dithiothreitol (DTT). The clearance rate against reaction time (min) and protein concentration (μg/mL) was monitored to evaluate the H2O2 reduction activity of CcPrx4 protein. All treatments were performed in triplicate, and data were shown as the mean ± SE (n = 3, * p < 0.05 vs. the control (heat-inactivated), ** p < 0.01 vs. the control). (B) Effects of CcPrx4 protein in protecting the supercoiled structure of pET-24a plasmid DNA against oxidative damage in the metal-catalyzed oxidation (MCO) system. Lane 1, pET-24a plasmid DNA only; lane 2, pET-24a plasmid DNA and FeCl3; lane 3, pET-24a plasmid DNA and DTT; lane 4, pET-24a plasmid DNA, FeCl3 and DTT; lane 5–10, pET-24a plasmid DNA, FeCl3, DTT and different concentrations of the purified CcPrx4 protein (200, 150, 100, 75, 50 and 25 μg/mL, respectively). The bands corresponding to the nicked form (NF) and the supercoiled form (SF) of the plasmid DNA are indicated on the right-hand side.