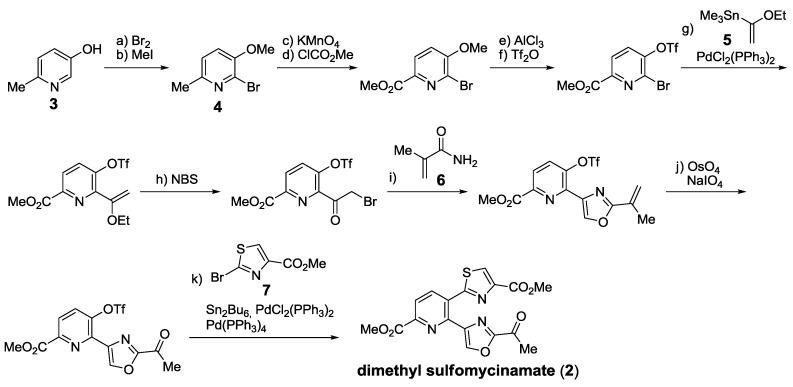
Scheme 5.
Kelly’s synthesis of dimethyl sulfomycinamate. Reagents and conditions: (a) Br2, pyridine, rt, 77%; (b) MeI, K2CO3, acetone, reflux, overnight 88%; (c) KMnO4, 90 °C, 3 h; (d) ClCO2Me, Et3N, DMAP, CH2Cl2, 0 °C to rt, 2 h, 65%; (e) AlCl3, CH2Cl2, reflux, 2 days, 93%; (f) Tf2O, 2,6-lutidine, CH2Cl2, 0 °C, 5 min, 95%; (g) 5, PdCl2(PPh3)2, 1,4-dioxane, 100 °C, overnight 97%; (h) NBS, THF, H2O, rt, 10 min, 95%; (i) 6, THF sealed tube, 100 °C, 3 days, 65%; (j) OsO4, NaIO4, 1,4-dioxane, H2O, rt 3 h, 85%; (k) 7, Sn2Bu6, PdCl2(PPh3)2, Pd(PPh3)4, LiCl, 1,4-dioxane, 100 °C, overnight, 35%.