Abstract
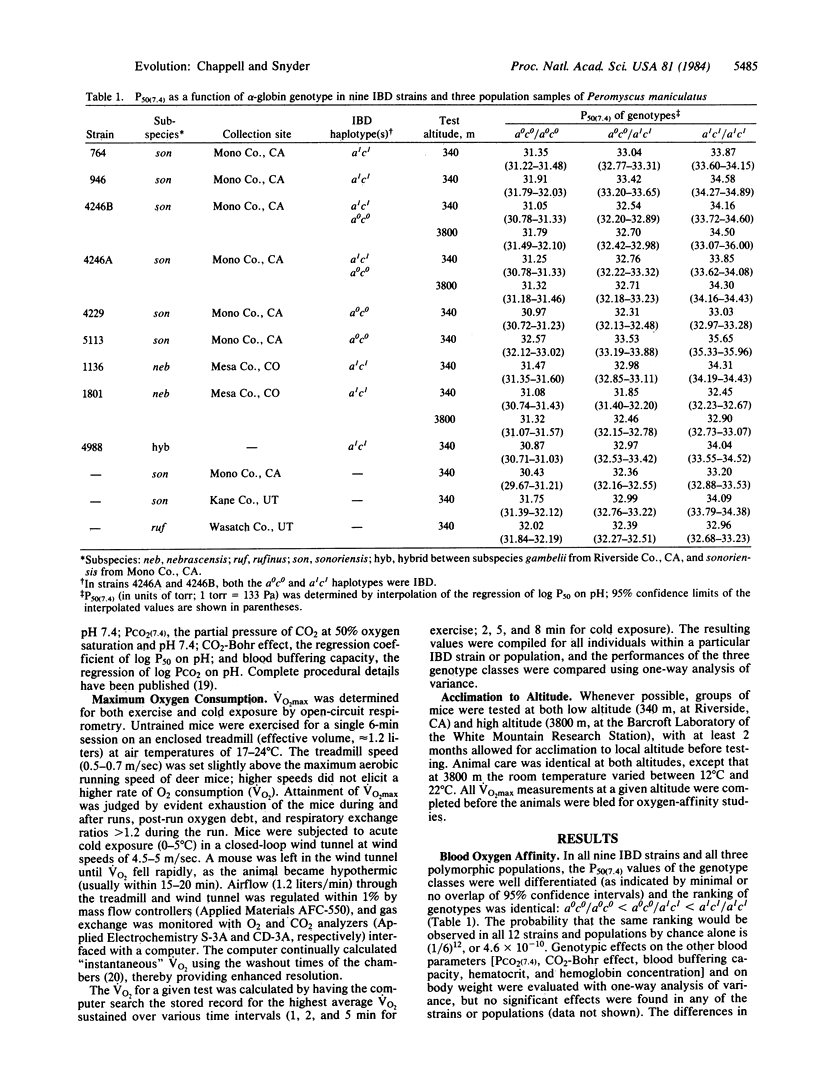
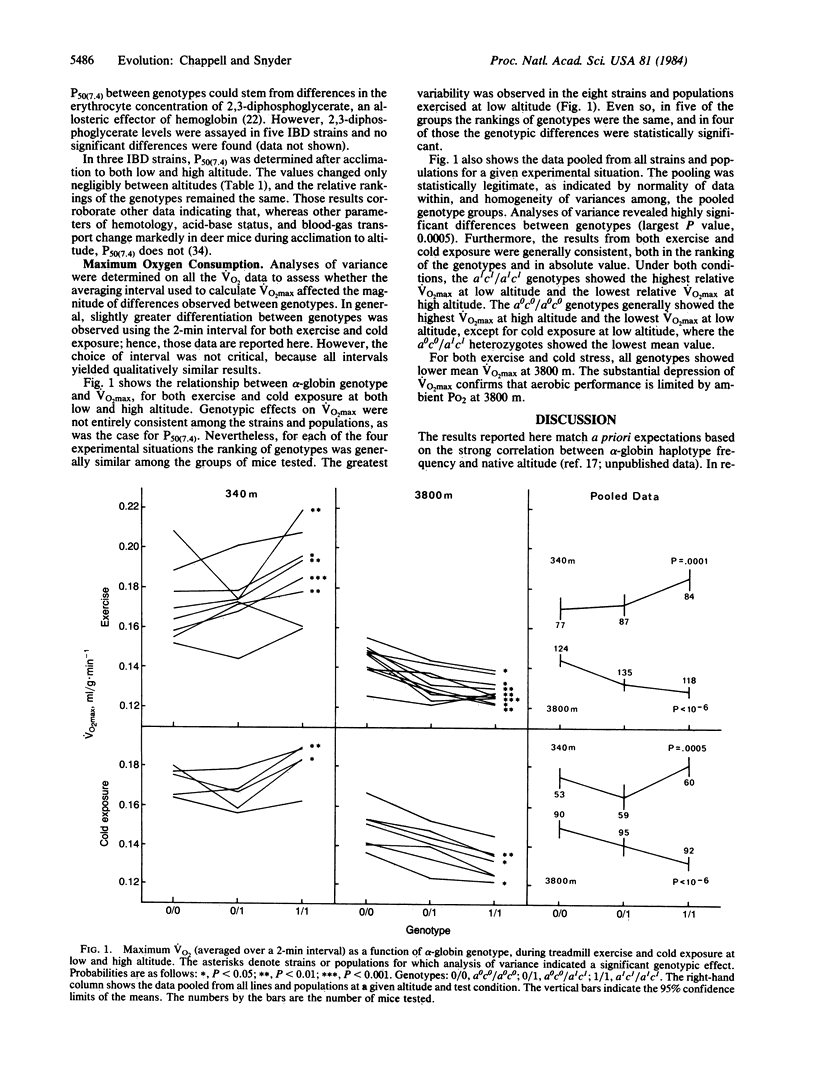
The alpha-hemoglobin chains in adult deer mice are usually encoded by two tightly linked loci. Because of strong linkage disequilibrium, almost all alpha-globin haplotypes fall into just two classes. The a0c0 class predominates in high-altitude populations, whereas the a1c1 class is generally fixed in low-altitude populations. Here we show that the alpha-globin genotype has effects at both the biochemical level [on blood oxygen affinity (P50)]and at the level of whole-animal physiology [on maximum rate of oxygen consumption (VO2max) during both exercise and cold exposure]. The a1c1/a1c1 genotype mice have the highest P50 values and show the highest Vo2max values at low altitude (340 m) but the lowest VO2max values at high altitude (3800 m). The a0c0/a0c0 mice have the lowest P50, and show the highest VO2max at high altitude but usually have the lowest VO2max at low altitude. The a0c0/a1c1 heterozygotes have an intermediate P50 and are generally intermediate in VO2max at both altitudes. Since high VO2max is advantageous for aerobic exercise and thermogenesis, the physiological data provide a potential explanation for the correlation of haplotype frequencies with altitude.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bencowitz H. Z., Wagner P. D., West J. B. Effect of change in P50 on exercise tolerance at high altitude: a theoretical study. J Appl Physiol Respir Environ Exerc Physiol. 1982 Dec;53(6):1487–1495. doi: 10.1152/jappl.1982.53.6.1487. [DOI] [PubMed] [Google Scholar]
- Brown A. J. Physiological correlates of an enzyme polymorphism. Nature. 1977 Oct 27;269(5631):803–804. doi: 10.1038/269803a0. [DOI] [PubMed] [Google Scholar]
- Burton R. S., Feldman M. W. Physiological effects of an allozyme polymorphism: glutamate-pyruvate transaminase and response to hyperosmotic stress in the copepod Tigriopus californicus. Biochem Genet. 1983 Apr;21(3-4):239–251. doi: 10.1007/BF00499136. [DOI] [PubMed] [Google Scholar]
- Cavener D. R., Clegg M. T. Evidence for biochemical and physiological differences between enzyme genotypes in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4444–4447. doi: 10.1073/pnas.78.7.4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMichele L., Powers D. A. LDH-B genotype-specific hatching times of Fundulus heteroclitus embryos. Nature. 1982 Apr 8;296(5857):563–564. doi: 10.1038/296563a0. [DOI] [PubMed] [Google Scholar]
- DiMichele L., Powers D. A. Physiological basis for swimming endurance differences between LDH-B genotypes of Fundulus heteroclitus. Science. 1982 May 28;216(4549):1014–1016. doi: 10.1126/science.7079747. [DOI] [PubMed] [Google Scholar]
- Eanes W. F. Viability interactions, in vivo activity and the G6PD polymorphism in Drosophila melanogaster. Genetics. 1984 Jan;106(1):95–107. doi: 10.1093/genetics/106.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. J., Martin R. J. Mixing technique for the oxygen-hemoglobin equilibrium and Bohr effect. J Appl Physiol. 1966 Nov;21(6):1898–1902. doi: 10.1152/jappl.1966.21.6.1898. [DOI] [PubMed] [Google Scholar]
- Hebbel R. P., Eaton J. W., Kronenberg R. S., Zanjani E. D., Moore L. G., Berger E. M. Human llamas: adaptation to altitude in subjects with high hemoglobin oxygen affinity. J Clin Invest. 1978 Sep;62(3):593–600. doi: 10.1172/JCI109165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbish T. J., Deaton L. E., Koehn R. K. Effect of an allozyme polymorphism on regulation of cell volume. Nature. 1982 Aug 12;298(5875):688–689. doi: 10.1038/298688a0. [DOI] [PubMed] [Google Scholar]
- Kavanau J. L. Behavior of captive white-footed mice. Science. 1967 Mar 31;155(3770):1623–1639. doi: 10.1126/science.155.3770.1623. [DOI] [PubMed] [Google Scholar]
- Luzzatto L., Usanga F. A., Reddy S. Glucose-6-phosphate dehydrogenase deficient red cells: resistance to infection by malarial parasites. Science. 1969 May 16;164(3881):839–842. doi: 10.1126/science.164.3881.839. [DOI] [PubMed] [Google Scholar]
- Moore L. G., Brewer G. J. Beneficial effect of rightward hemoglobin-oxygen dissociation curve shift for short-term high-altitude adaptation. J Lab Clin Med. 1981 Jul;98(1):145–154. [PubMed] [Google Scholar]
- Sampsell B., Sims S. Effect of adh genotype and heat stress on alcohol tolerance in Drosophila melanogaster. Nature. 1982 Apr 29;296(5860):853–855. doi: 10.1038/296853a0. [DOI] [PubMed] [Google Scholar]
- Snyder L. R. 2,3-diphosphoglycerate in high- and low-altitude populations of the deer mouse. Respir Physiol. 1982 Apr;48(1):107–123. doi: 10.1016/0034-5687(82)90053-6. [DOI] [PubMed] [Google Scholar]
- Snyder L. R., Born S., Lechner A. J. Blood oxygen affinity in high- and low-altitude populations of the deer mouse. Respir Physiol. 1982 Apr;48(1):89–105. doi: 10.1016/0034-5687(82)90052-4. [DOI] [PubMed] [Google Scholar]
- Snyder L. R. Genetics of hemoglobin in the deer mouse, Peromyscus maniculatus. I. Multiple alpha- and beta-globin structural loci. Genetics. 1978 Jul;89(3):511–530. doi: 10.1093/genetics/89.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder L. R. Genetics of hemoglobin in the deer mouse, Peromyscus maniculatus. II. Multiple alleles at regulatory loci. Genetics. 1978 Jul;89(3):531–550. doi: 10.1093/genetics/89.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek Z., Kreuzer F., Hoofd L. J. Advantage or disadvantage of a decrease of blood oxygen affinity for tissue oxygen supply at hypoxia. A theoretical study comparing man and rat. Pflugers Arch. 1973 Aug 27;342(3):185–197. doi: 10.1007/BF00591367. [DOI] [PubMed] [Google Scholar]
- Watt W. B., Cassin R. C., Swan M. S. Adaptation at Specific Loci. III. Field Behavior and Survivorship Differences among Colias Pgi Genotypes Are Predictable from IN VITRO Biochemistry. Genetics. 1983 Apr;103(4):725–739. doi: 10.1093/genetics/103.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]


