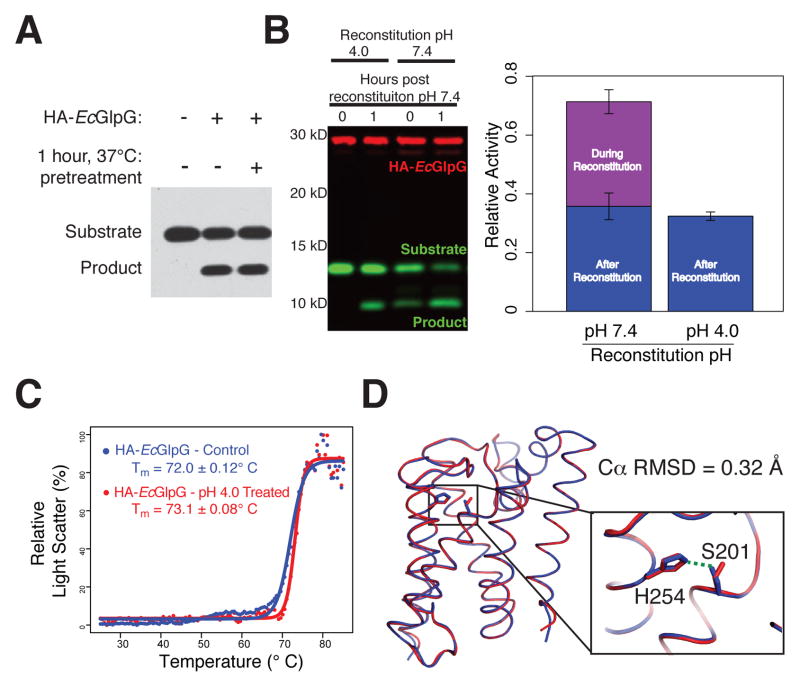
Figure 1. An inducible co-reconstitution system for membrane enzyme analysis.
(A) HA-tagged GlpG (HA-EcGlpG) preincubated for 1 hour at 37°C retained full activity against the substrate APP+Spi7-Flag (shown is an anti-Flag western). (B) HA-EcGlpG was inactive during the reconstitution with APP+Spi7-Flag at pH 4, but active when reconstituted at pH 7.4 (t=0 reaction times). Activity was restored upon neutralization to pH 7.4 (see 1h reaction time). Shown is a 2-color western, and quantification (graph) revealing the amount of protease activity in proteoliposomes was indistinguishable whether the protease was subjected to pH shift or not. (C) Thermostability of HA-EcGlpG without and after pretreatment in pH 4 buffer was examined by heating from 25 to 85°C and monitoring differential static light scattering every 0.5°C. (D) X-ray crystal structure comparison of ΔN-EcGlpG at low (red) and neutral (blue) pH. Note that although the overall conformation is indistinguishable (Cα RMSD = 0.32 Å), at low pH the catalytic serine 201 sidechain was no longer hydrogen-bonded to the histidine base (inset), rendering the enzyme catalytically inactive. See Table S1 for structural parameters.