Figure 6.
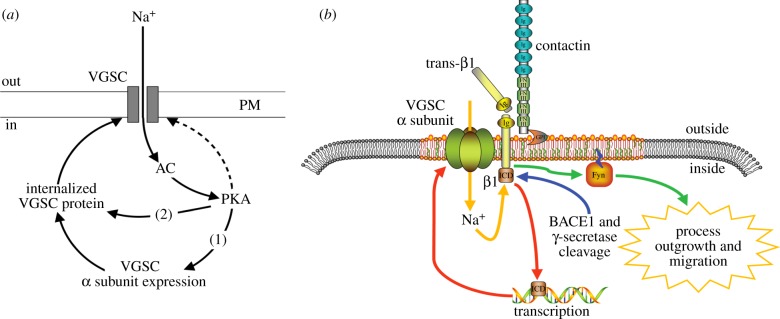
Activity-dependent regulation of VGSC expression and VGSC-dependent migration. (a) In Mat-LyLu [28] and MDA-MB-231 [34] cells, INa activates adenylate cyclase (AC) and protein kinase A (PKA), which in turn (i) potentiates α-subunit mRNA expression and (ii) increases channel expression at the plasma membrane, without affecting total cellular α-subunit protein level. PKA also directly phosphorylates surface-expressed VGSCs, although this may be independent of PKA (dashed line). Adapted from [28]. (b) Interplay between α and β1 subunits in transcription and process outgrowth, modelled from cerebellar granule neurons. Trans adhesion between β1 on an adjacent cell and a VGSC signalling complex (comprising α, β1 subunits and contactin), initiates a signalling cascade via FYN kinase that enhances process outgrowth and migration. Proteolytic processing of β1 by BACE1 and γ-secretase is proposed to release the soluble intracellular domain of β1, which may in turn enhance transcription of α subunit genes. Nav1.6 activity is required for β1-mediated process outgrowth, and in turn, β1 is required for normal localization of α-subunits. Thus, INa may fine-tune the dual processes of gene expression and migration. Adapted from [117]. (Online version in colour.)
