Abstract
Rationale: β2-Agonists are the treatment of choice for exercise-induced bronchoconstriction (EIB) and act through specific receptors (ADRB2). Arg16Gly polymorphisms have been shown to affect responses to regular use of β2-agonists.
Objectives: To evaluate the influence of the Arg16Gly receptor polymorphism on salmeterol bronchoprotection in EIB and assess predictors of bronchoprotection.
Methods: A prospective, genotype-blinded, randomized trial was performed in 26 subjects (12 Arg16Arg and 14 Gly16Gly) with EIB who were not on controller therapy. Subjects were administered salmeterol, 50 μg twice a day for 2 weeks, and underwent an exercise challenge 9 hours after the first and last drug dose. In addition to genotype, FEV1, response to salmeterol, degree of EIB, and exhaled nitric oxide (FeNO) at baseline were examined for their association with loss of bronchoprotection (LOB).
Measurements and Main Results: The maximum exercise-induced FEV1 fall was 27.9 ± 1.4% during the run-in period, 8.1 ± 1.2% (70.3 ± 4.1% bronchoprotection) after the first salmeterol dose, and 22.8 ± 3.2% (18.9 ± 11.5% bronchoprotection) after 2 weeks of salmeterol (P = 0.0001). The Arg16Gly polymorphisms were not associated with the LOB in response to salmeterol. FeNO values at baseline were significantly related to the LOB (r = 0.47; P = 0.01). Mean change was a 74 ± 13% LOB in subjects with FeNO levels greater than 50 ppb and a 7 ± 16% gain in bronchoprotection in those with FeNO levels less than 25 ppb (P = 0.01).
Conclusions: The LOB that occurs with chronic long-acting β2-agonists use is not affected by ADRB2 Arg16Gly polymorphisms. High FeNO was associated with marked LOB. Use of long-acting β2-agonists before achieving a reduction in FeNO may need to be avoided.
Clinical trial registered with www.clinicaltrials.gov (NCT 00595361).
Keywords: asthma, β2-agonist, nitric oxide, pharmacogenetics, tolerance
At a Glance Commentary
Scientific Knowledge on the Subject
ADRB2 polymorphisms have been reported to influence the response to β2-agonists because of receptor down-regulation in subjects with asthma. No pharmacogenetic data are available, at present, in exercise-induced bronchoconstriction.
What This Study Adds to the Field
This study shows that the Arg16Gly polymorphism does not influence the bronchoprotective effect of regular salmeterol treatment in exercise-induced bronchoconstriction. Furthermore, results obtained demonstrate that the loss of bronchoprotection against exercise, observed after 2 weeks of salmeterol treatment, can be predicted by high exhaled nitric oxide levels.
Inhaled β2-adrenergic agonists are widely used and have been proved to be the most effective treatment available to acutely prevent and reverse the bronchial obstruction that occurs after physical activity (1). Both short-acting and long-acting β2-agonists (SABA and LABA) administered in a standard dose immediately before exercise have been shown to reduce the fall in FEV1 by 70–80% most subjects (2).
However, daily treatment may lead to tolerance to the bronchoprotective effect (3). This effect has been documented in response to direct (methacholine and adenosine monophosphate [AMP]) (4) and indirect (allergen and exercise) stimuli (5, 6), occurring as early as 7 days after regular use and even in the face of concomitant use of inhaled corticosteroids (ICS) (3). The loss of bronchoprotection (LOB) has been reported to range from 49% to 72% of the initial observed effect (3) with some subjects retaining near complete bronchoprotection and others developing a paradoxical increase in bronchoconstriction to the provocative stimulus.
It has been speculated this phenomenon may be caused by receptor down-regulation, reduced production or internalization of receptors, and uncoupling from secondary messengers (7). However, the precise mechanism of the LOB is unclear. Polymorphisms at the 16th amino acid position of the β2-adrenergic receptor (Arg16Gly ADRB2) have been shown to affect receptor down-regulation (8). Arg16Arg homozygous patients have been shown to have reduced airway caliber and increased symptoms when using short-acting β-agonists regularly (9, 10). Although regular LABAs, used concomitantly with moderate- to high-dose ICS, did not produce a reduction in airway caliber in Arg16Arg patients, it did result in an increase in airway reactivity to methacholine in Arg16Arg subjects compared with Gly16Gly patients (11). In a retrospective analysis Lee and coworkers (12) suggested that subjects with an Arg allele had reduced bronchoprotection to methacholine and AMP. Furthermore, Snyder and coworkers (13) showed that during recovery after exercise, the Arg16Arg genotype is associated with reduced bronchodilation in healthy adults.
Because prior studies had been retrospective and assessed direct challenges, we designed a prospective trial in which we examined the influence of the Arg16Gly polymorphism on the LOB to salmeterol against airway narrowing produced by exercise. In addition to variation in ADRB2, airway inflammation has been postulated to interfere with β-receptor function in asthma (14). Therefore, we also sought to examine whether baseline functional and inflammatory parameters could influence the Arg16Gly pharmacogenetic effect.
Methods
Study Design
A double-blind (to genotype) prospective cohort study was conducted at the Asthma Research Center of the Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts and at the Department of Asthma, Allergy and Pulmonary Clinical Research of the University of Wisconsin, School of Medicine and Public Health, Madison, Wisconsin. The study was approved by the Partners Human Research and the University of Wisconsin Health Sciences Institutional Review Boards and was registered on clinicaltrials.gov (NCT 00595361).
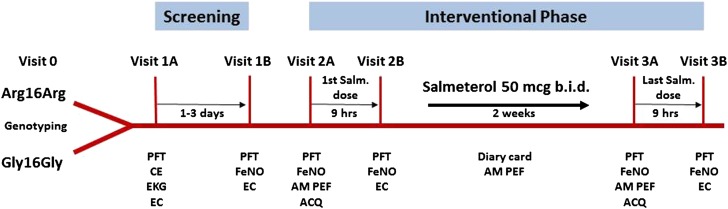
The protocol included a total of seven visits: one preliminary screening, two screening, and four study visits (Figure 1). The preliminary screening visit (visit 0) consisted of collecting a blood or saliva sample for genotyping. Subjects homozygous for the Arg/Arg or Gly/Gly polymorphism at codon 16 of the β2-adrenergic receptor were invited to return for the first screening visit (visit 1A). Those with exercise-induced bronchoconstriction (EIB), documented by a positive response to the exercise challenge (FEV1 fall ≥20%) were reevaluated within 1–3 days (visit 1B). Subjects who demonstrated a positive response to this second confirmatory exercise challenge (FEV1 fall not ≤18%) were enrolled into the interventional phase (visits 2A–3A) and were administered salmeterol, 50 μg puff twice a day for 2 weeks. The treatment duration was chosen to be adequate to show tachyphylaxis (6), if any, while reducing as much as possible subjects’ exposure to LABA monotherapy. Nine hours after visits 2A and 3A the bronchoprotective effect on EIB of the first and the last salmeterol dose, respectively, was evaluated through an exercise challenge (visits 2B and 3B).
Figure 1.
Study flowchart. ACQ = Asthma Control Questionnaire; AM PEF = morning peak expiratory flow; CE = clinical examination; EC = exercise challenge; EKG = electrocardiography; FeNO = exhaled nitric oxide; PFT = pulmonary function tests.
Outcome variables included pulmonary function tests, exhaled nitric oxide (FeNO), asthma control questionnaire (ACQ; seven items) and clinical symptoms (Figure 1).
Study Population
Male and female subjects with asthma between 18 and 50 years of age, with a baseline FEV1 greater than or equal to 65% of predicted, a positive history of EIB, and not on controller medications were eligible for the study. Exclusion criteria included cardiac or concomitant respiratory diseases, smoking history greater than or equal to 10 pack-years or smoking within the past 12 months, asthma exacerbations requiring treatment changes, upper respiratory tract infections, or use of systemic corticosteroids within the previous 4 weeks. All patients provided written informed consent.
Study Drug
Salmeterol, 50 μg, was administered through a dry powder inhaler (Serevent Diskus; GlaxoSmithKline, Brentford, UK) twice a day for 2 weeks.
Study Procedures
Genotyping.
Genotyping was performed in a blinded manner at a central laboratory using blood or saliva samples collected during the prescreening visit. At amino acid 16 of the β2-adrenergic receptor, the three possible genotypes Arg16Arg, Arg16Gly, and Gly16Gly were assessed by the TaqMan allelic discrimination assay method. Genotype results were then confirmed in all enrolled subjects by DNA sequencing.
Clinical history and physical examination.
Clinical history was obtained with special reference to smoking habits; asthma symptoms; systemic, cardiovascular, and concomitant respiratory diseases; and past and current treatments. General physical examination included height, weight, body temperature, and arterial blood pressure measurement; electrocardiography; and pregnancy test in females of childbearing age.
Pulmonary function tests.
Pulmonary function tests were performed according to the American Thoracic Society (ATS) guidelines (15) at baseline, at 9 hours after salmeterol administration, and at different time-points after the exercise challenge (1, 3, 5, 10, 20, 30, 45, 60 min). The best of three attempts was recorded at each time point.
The maximum FEV1 percentage fall after exercise was calculated according to the formula:
The degree of bronchoprotection offered by the first and the last salmeterol dose was expressed according the following formulas, respectively:
Exercise challenge.
Exercise testing protocol was performed according to the ATS guidelines (16). Subjects were instructed to run for 6–8 minutes on a treadmill while inhaling dry air at room temperature from compressed tanks. A workload that increased the heart rate to 80–90% of the subject’s age-predicted maximum (220 − age) was reached within the first 2 minutes and was maintained until the end of the challenge. Subjects had to demonstrate evidence of EIB, as defined by a maximum FEV1 percentage fall greater than or equal to 20% at the first screening visit and not less than 18% (to allow for test variability) on a confirmatory exercise challenge.
Exhaled nitric oxide.
FeNO was measured online by chemiluminescence at a constant expiratory flow (50 ml/s), consistent with published guidelines (17). The mean value of two consecutive valid measurements was recorded.
Asthma control questionnaire.
The degree of asthma control was evaluated through the standardized seven-item ACQ (18).
Clinical symptoms, PEF, and use of rescue medications.
Subjects were given a peak-flow meter and carefully instructed by the study investigator to record the following information on a diary card daily, during the 2-week treatment period: presence of clinical symptoms (cough, chest tightness, wheezing, and shortness of breath) scored according to severity from 0 to 3, number of nocturnal awakenings, use of rescue medications (number of puffs of albuterol), and morning PEF measurements.
Study Outcomes
Study outcomes were evaluated at the end of the 2-week treatment period and reported as changes from baseline to the last salmeterol dose in Arg16Arg compared with Gly16Gly subjects. The primary endpoint was represented by the maximum FEV1 percentage fall. Secondary endpoints were FEV1 at baseline and 9 hours after administration of salmeterol dose, morning PEF, ACQ, and FeNO.
Data Analysis
Statistical analysis was performed using SPSS 20.0 software (IBM Corporation, Armonk, NY). Data were expressed as mean ± SE. Student t test was used to compare continuous variables in the two different groups. Binominal variables were analyzed using the chi-squared test. Correlations between variables were assessed using the Pearson correlation coefficient. A P value less than 0.05 was considered statistically significant. With a power set to 80%, α error of 5%, and standard deviation of 9%, 26 subjects were required to detect a treatment difference of 10% in the primary outcome.
Results
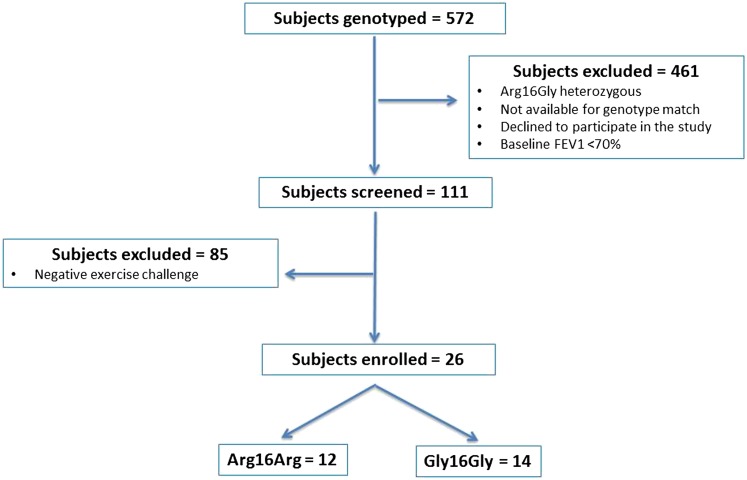
Among 572 subjects genotyped, 111 subjects were found to be homozygous for Arg16Arg or Gly16Gly and were screened to document EIB. The first 26 consecutive subjects fulfilling inclusion criteria were enrolled into the treatment phase (Figure 2).
Figure 2.
CONSORT diagram.
There were 20 female subjects. Mean age was 26.6 years, mean baseline FEV1 (% predicted) was 83.6 ± 1.8%, and mean maximum FEV1 % fall after screening exercise challenges was 27.9 ± 1.4%. No significant differences were observed for the above considered variables in the two groups according to the Arg16Gly polymorphism (Table 1).
TABLE 1.
STUDY POPULATION AT SCREENING
| Study Population | Arg16Arg | Gly16Gly | P Value* | |
|---|---|---|---|---|
| Number of subjects | 26 | 12 | 14 | ns |
| Sex, M/F | 6/20 | 2/10 | 4/10 | ns |
| Age, yr | 26.6 ± 1.3 | 25.8 ± 2.4 | 27.2 ± 1.4 | ns |
| Race | 18W, 3H, 3B, 1A, 1O | 7W, 3B, 1H, 1A | 11W, 2H, 1O | ns |
| Baseline FEV1, % predicted | 83.6 ± 1.8 | 80.3 ± 2.7 | 86.4 ± 2.5 | ns |
| Max FEV1 % fall after exercise | 27.9 ± 1.4 | 26.1 ± 1.9 | 29.4 ± 1.8 | ns |
Definition of abbreviations: A = Asian; B = black; H = Hispanic; ns = not significant; O = others; W = white.
Data are expressed as mean ± SE.
Comparison between genotypes.
Characteristics of the entire study population and by genotype at the beginning of the interventional phase (visit 2) are reported in Table 2. After the first salmeterol dose, the FEV1 at 9 hours increased by 9.3 ± 1.2%. The maximum fall in FEV1 after exercise was 8.1 ± 1.2%, representing a bronchoprotection of 70.3 ± 4.1% compared with the baseline fall. At the beginning of treatment, Arg16Arg and Gly16Gly subjects were well matched in terms of baseline FEV1, morning PEF, FeNO, ACQ, and FEV1 at 9 hours after the first administration of salmeterol (Table 2). The maximal percent fall in FEV1 after the first exercise challenge did not differ between genotypes (7.6 ± 1.6% in Arg16Arg and 8.6 ± 1.7% in Gly16Gly; P = 0.48).
TABLE 2.
CHARACTERISTICS OF THE ENTIRE STUDY POPULATION AND BY GENOTYPE AT THE BEGINNING OF SALMETEROL TREATMENT PERIOD (VISIT 2)
| Study Population (n = 26) | Arg16Arg (n = 12) | Gly16Gly (n = 14) | P Value* | |
|---|---|---|---|---|
| FEV1 preexercise, % predicted | 82.0 ± 1.8 | 79.9 ± 2.6 | 83.8 ± 2.5 | 0.29 |
| Morning PEF, L/min | 406.4 ± 20.2 | 370.4 ± 22.2 | 439.6 ± 31.2 | 0.08 |
| ACQ | 1.2 ± 0.1 | 1.3 ± 0.2 | 1.1 ± 0.2 | 0.31 |
| FeNO, ppb | 53.3 ± 6.8 | 47.6 ± 9.4 | 58.2 ± 9.9 | 0.44 |
| FEV1 9 h after salmeterol, % predicted | 91.3 ± 1.9 | 90.5 ± 2.5 | 92.0 ± 2.9 | 0.70 |
| FEV1 9 h after salmeterol, % increase from preexercise | 9.3 ± 1.2 | 10.5 ± 1.7 | 8.2 ± 1.7 | 0.35 |
| Max FEV1 % fall after exercise | 8.1 ± 1.2 | 7.6 ± 1.6 | 8.6 ± 1.7 | 0.68 |
| % Bronchoprotection | 70.3 ± 4.1 | 69.6 ± 7.0 | 70.9 ± 5.0 | 0.87 |
Definition of abbreviations: ACQ = asthma control questionnaire; FeNO = exhaled nitric oxide.
Data are expressed as mean ± SE.
Comparison between genotypes.
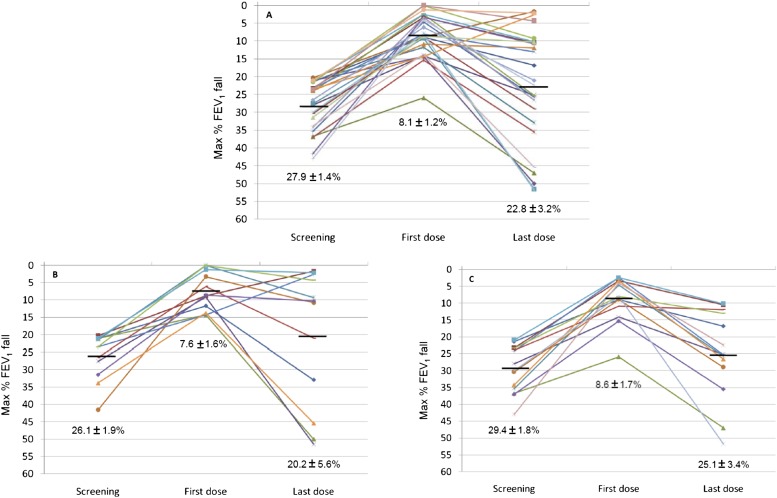
After 2 weeks of salmeterol treatment (Table 3), the 26 subjects showed a significant increase in morning PEF (from 406.4 ± 20.2 to 445.0 ± 19.1; P = 0.00003). Although not significant, there was a trend toward improvement in most of the other endpoints considered (baseline FEV1, ACQ score, FeNO). The % increase from the baseline value in FEV1 assessed 9 hours after the salmeterol dose was significantly reduced from 9.3 ± 1.2 at the beginning of the treatment period to 3.5 ± 1.2 at the end of the interventional phase (P = 0.002). The mean fall in FEV1 postexercise was 22.8 ± 3.2%. This fall was significantly higher (P = 0.00001) than that recorded after the first salmeterol dose (8.1 ± 1.2%) and comparable with that at screening (27.9 ± 1.4%) (Figure 3; individual data are reported in the online supplement). After 2 weeks of salmeterol the mean inhibition of the maximal fall in FEV1 was only 18.9 ± 11.5% compared with 70.3 ± 4.1% at the start of treatment (P = 0.0001). Twenty-one of 26 subjects experienced a significant (>10%) LOB. Seven subjects experienced even a worsening of EIB (greater fall in FEV1 than they had experienced without any salmeterol treatment) after regular salmeterol use (Figure 3).
TABLE 3.
PRIMARY AND SECONDARY OUTCOMES IN THE ENTIRE STUDY POPULATION AT THE END OF THE SALMETEROL TREATMENT PERIOD (VISIT 3) AND AS CHANGE FROM VISIT 2
| Outcomes | End of Treatment (n = 26) | Change (V2 to V3) (n = 26) | P Value* |
|---|---|---|---|
| FEV1 preexercise, % predicted | 87.0 ± 2.3 | 4.5 ± 1.8 | 0.09 |
| Morning PEF, L/min | 445.0 ± 19.1 | 38.6 ± 7.6 | 0.00003 |
| ACQ | 0.8 ± 0.1 | −0.4 ± 0.1 | 0.057 |
| FeNO, ppb | 45.3 ± 5.6 | −6.1 ± 4.3 | 0.36 |
| FEV1 9 h after salmeterol, % predicted | 90.2 ± 2.1 | −1.5 ± 0.9 | 0.71 |
| FEV1 9 h after salmeterol, % increase from preexercise | 3.5 ± 1.2 | −6.0 ± 1.6 | 0.002 |
| Max FEV1 % fall after exercise | 22.8 ± 3.2 | 14.7 ± 2.7 | 0.00001 |
| % Bronchoprotection | 18.9 ± 11.5 | −51.4 ± 10.2 | 0.0001 |
Definition of abbreviations: ACQ = asthma control questionnaire; FeNO = exhaled nitric oxide.
Data are expressed as mean ± SE.
Comparison between the beginning and the end of treatment.
Figure 3.
Maximal FEV1 % fall (A) in the study population and (B, C) by genotype (B, Arg16Arg; C, Gly16Gly). Data are expressed as mean ± SE.
At the end of the treatment period, Gly16Gly subjects had significantly higher morning PEF values compared with Arg16Arg subjects (483.1 ± 30.8 vs. 403.8 ± 17.5; P = 0.04). The degree of bronchoprotection and all other endpoints did not differ between the genotypes after 2 weeks of salmeterol (Table 4). Changes in outcomes considered were not significantly influenced by the Arg16Gly ADRB2 polymorphism (Table 4). The mean maximal FEV1 % fall after 2 weeks of salmeterol similarly increased both in Arg16Arg and in Gly16Gly subjects (+12.6 ± 4.5% and +16.5 ± 2.9%, respectively; P > 0.05). These falls represented a 48.1 ± 20.3% and a 54.3 ± 9.3% LOB in Arg16Arg versus Gly16Gly subjects (P = 0.77).
TABLE 4.
PRIMARY AND SECONDARY OUTCOMES BY GENOTYPE AT THE END OF THE SALMETEROL TREATMENT PERIOD (VISIT 3) AND AS CHANGE FROM VISIT 2
| Outcomes | End of Treatment Arg16Arg (n = 12) | End of Treatment Gly16Gly (n = 14) | P Value* | Change (V2 to V3) Arg16Arg (n = 12) | Change (V2 to V3) Gly16Gly (n = 14) | P Value† |
|---|---|---|---|---|---|---|
| FEV1 preexercise, % predicted | 84.7 ± 3.6 | 89.0 ± 3.1 | 0.37 | 4.2 ± 2.7 | 4.6 ± 2.6 | 0.91 |
| Morning PEF, L/min | 403.8 ± 17.5 | 483.1 ± 30.8 | 0.04 | 33.3 ± 7.0 | 43.5 ± 13.2 | 0.51 |
| ACQ | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.88 | −0.5 ± 0.2 | −0.2 ± 0.2 | 0.10 |
| FeNO, ppb | 46.6 ± 29.3 | 44.0 ± 7.6 | 0.77 | −1.0 ± 5.5 | −10.9 ± 6.5 | 0.26 |
| FEV1 9 h after salmeterol, % predicted | 88.1 ± 2.8 | 91.9 ± 3.1 | 0.38 | −3.3 ± 1.3 | −0.1 ± 1.3 | 0.10 |
| FEV1 9 h after salmeterol, % increase from preexercise | 3.4 ± 1.9 | 3.5 ± 1.8 | 0.94 | −7.4 ± 2.7 | −4.6 ± 2.2 | 0.43 |
| Max FEV1 % fall | 20.2 ± 5.6 | 25.1 ± 3.4 | 0.45 | 12.6 ± 4.5 | 16.5 ± 2.9 | 0.48 |
| % Bronchoprotection | 21.5 ± 23.4 | 16.6 ± 9.6 | 0.84 | −48.1 ± 20.3 | −54.3 ± 9.3 | 0.77 |
Definition of abbreviations: ACQ = asthma control questionnaire; FeNO = exhaled nitric oxide.
Data are expressed as mean ± SE.
Comparison between genotypes in absolute values at the end of treatment.
Comparison between genotypes as change from the beginning to the end of treatment.
Degree of LOB did not associate with baseline airway tone (r = 0.16; P = 0.43), bronchodilator response to salmeterol (r = −0.33; P = 0.09), or degree of responsiveness to exercise (r = −0.24; P = 0.22), assessed as baseline FEV1, FEV1 9 hours after salmeterol, and maximum FEV1 % fall, respectively.
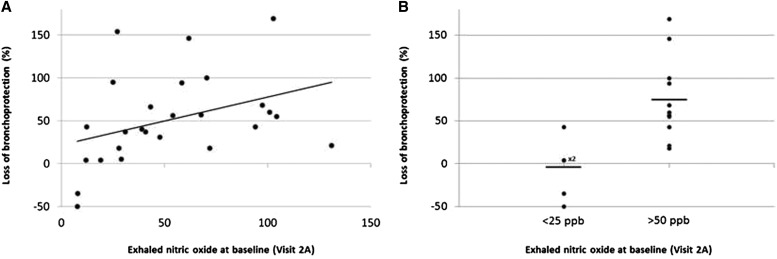
Baseline FeNO significantly correlated with the LOB (r = 0.47; P = 0.01) (Figure 4A). High levels of FeNO seemed to be a valuable predictive marker for the onset of LOB. Expressing data as absolute % change from the first to the last salmeterol dose (Figure 4B), subjects with FeNO greater than 50 ppb (n = 12) showed a LOB significantly greater than those (n = 5) with FeNO less than 25 ppb (74% vs. −7%; P = 0.01).
Figure 4.
(A) Correlation between exhaled nitric oxide baseline values and loss of bronchoprotection. P = 0.01; r = −0.47. (B) Loss of bronchoprotection in subjects with exhaled nitric oxide values less than 25 ppb (n = 5; ×2 = two subjects with the same loss of bronchoprotection value) and greater than 50 ppb (n = 12). P = 0.01.
Discussion
Although the ADRB2 Arg16Gly polymorphism influences the clinical response to regular use of β2-agonists, we did not find that it was associated with differences in LOB to salmeterol. However, airway inflammation, as assessed by baseline FeNO, was significantly associated with LOB from regular salmeterol use.
In this trial, regular LABA administration induced a marked tolerance to the bronchoprotective effect against EIB, when assessed 9 hours after salmeterol administration. In fact, the 2-week salmeterol treatment period caused, on average, an almost complete LOB. A variable degree of LOB, after β2-agonist administration, has also been reported by others, depending on the drug used and the time-point chosen for the assessment (6, 19). However, our study design does not allow us to determine if a similar LOB would have been observed even choosing a different time interval between the salmeterol administration and the exercise challenge. The high female prevalence (20 out of 26) in our population sample, although notable, does not permit any speculation on a potential gender effect on the LOB.
Although regular use of short-acting β-agonists has been shown to reduce the efficacy of these agents in Arg16Arg patients compared with Gly16Gly subjects (9, 10), and the use of LABAs was associated with increased methacholine responsiveness (11) and increased LOB to direct challenges (12), we did not find a difference in the loss of effectiveness of salmeterol in protecting against EIB between these subjects. Furthermore, we did not note differences in baseline lung function, responsiveness to salmeterol, or degree of response to exercise between the genotypes.
In contrast to the retrospective report of Lee and coworkers (12), we did not find a genotype-related LOB. Several factors may account for this difference. We used exercise as opposed to pharmacologic inducers of bronchoconstriction. Thus, it is possible that the mechanisms of LOB may differ among these bronchoprovocative stimuli. Furthermore, we examined salmeterol alone, whereas those investigators referred to studies using salmeterol or formoterol in corticosteroid-treated patients with asthma. Lastly, our subjects were screened to have significant degree of induced bronchospasm, which may have obscured a finer degree of LOB.
We did observe that the morning PEF values were significantly higher at the end of treatment in Gly16Gly subjects. However, morning PEF was also higher in Gly16Gly subjects at baseline. Although other reports have suggested a trend toward higher baseline lung function in Gly16Gly subjects (20), in our case, the higher baseline lung function may be caused by the higher number of males in the Gly16Gly group.
Although we did not find a relationship between the candidate ADRB2 polymorphisms and LOB, we did find a significant association between FeNO baseline values and the loss of the bronchoprotective effect of a LABA. Importantly, when subjects were grouped according to the recommendations of the recent ATS guidelines on FeNO (21) (>50 and <25) those with values greater than 50 ppb showed a significantly higher LOB, when compared with those with values less than 25 ppb. In particular, we found that for patients with EIB who had a FeNO greater than 50, 9 of 12 had a greater than 50% LOB.
To our knowledge, this is the first study to show a significant correlation between baseline FeNO levels and LOB to exercise while on regular LABA monotherapy. Others have shown that elevated FeNO is associated with a higher likelihood of EIB (22, 23). Although elevated FeNO cannot be considered as a direct cause of increased LOB, it is of interest to speculate on their association. Levels higher than 50 ppb of FeNO have been reported to be associated with glucocorticoid-responsive eosinophilic airway inflammation (24). Additionally, in inflamed airways, cellular production of nitric oxide is associated with simultaneous superoxide anion (.O2-) production (25). The latter results in the formation of peroxynitrite, which has been shown to cause significant impairment in the bronchoprotective effect of isoprenaline in a dose-dependent manner in the airway smooth muscle (25). These effects are believed to be mediated through adenylate cyclase activity or downstream of such activity.
Our finding suggests that individuals with asthma with elevated baseline FeNO levels may not be appropriate candidates for LABA treatment until their underlying airway inflammation is addressed. Because none of our patients were on ICS, it is possible that these findings are not applicable in the setting of ICS use. However, waning of LABA-induced bronchoprotection with regular use occurs even in the setting of ICS use (3, 12), suggesting that the phenomenon we observed may not be altered by concomitant ICS. Whether this is caused by persistently elevated FeNO levels while taking steroids, nonadherence to ICS treatment, or noneosinophilic airway inflammation represents an issue for future investigation. Furthermore, although it is not generally recommended that LABAs be used without ICS, a survey among athletes revealed that 25% of them are using LABAs as monotherapy (26). Lastly, although it could be argued that patients with EIB should be treated with regular concomitant ICS, studies examining patients with EIB suggest that this condition is associated with elevated FeNO levels. Nonetheless, our study, performed in a highly selected sample, would benefit by replication on a general population on ICS.
In conclusion, our study shows that the bronchoprotective effect of regular LABA treatment on EIB is not influenced by the ADRB2 Arg16Gly polymorphism. However, 2 weeks of salmeterol treatment induces a marked LOB, which may be predicted by high FeNO levels. Our findings suggest that the use of LABAs in such patients should be reconsidered until the underlying airway inflammation is better controlled.
Acknowledgments
Acknowledgment
The authors thank Gautham Marigowda for his assistance as Laboratory Manager.
Footnotes
Supported by Informative Clinical Studies in Asthma (National Institutes of Health/NHLBI U10 HL74227), Research Skills Development Core Supplement (National Institutes of Health/NHLBI U10 HL51831), and ERS Research Fellowship (M.B.).
Author Contributions: All authors significantly contributed to study conception and design; data acquisition, analysis, and interpretation; drafting the article or revising it critically; and final approval of the version to be published.
Originally Published in Press as DOI: 10.1164/rccm.201307-1323OC on November 14, 2013
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Weiler JM, Anderson SD, Randolph C, Bonini S, Craig TJ, Pearlman DS, Rundell KW, Silvers WS, Storms WW, Bernstein DI, et al. Pathogenesis, prevalence, diagnosis, and management of exercise-induced bronchoconstriction: a practice parameter. Ann Allergy Asthma Immunol. 2010;105(Suppl 6):S1–S47. doi: 10.1016/j.anai.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 2.Anderson SD, Caillaud C, Brannan JD. Beta2-agonists and exercise-induced asthma. Clin Rev Allergy Immunol. 2006;31:163–180. doi: 10.1385/CRIAI:31:2:163. [DOI] [PubMed] [Google Scholar]
- 3.Salpeter SR, Ormiston TM, Salpeter EE. Meta-analysis: respiratory tolerance to regular beta2-agonist use in patients with asthma. Ann Intern Med. 2004;140:802–813. doi: 10.7326/0003-4819-140-10-200405180-00010. [DOI] [PubMed] [Google Scholar]
- 4.Jokic R, Swystun VA, Davis BE, Cockcroft DW. Regular inhaled salbutamol: effect on airway responsiveness to methacholine and adenosine 5′-monophosphate and tolerance to bronchoprotection. Chest. 2001;119:370–375. doi: 10.1378/chest.119.2.370. [DOI] [PubMed] [Google Scholar]
- 5.Cockcroft DW, McParland CP, Britto SA, Swystun VA, Rutherford BC. Regular inhaled salbutamol and airway responsiveness to allergen. Lancet. 1993;342:833–837. doi: 10.1016/0140-6736(93)92695-p. [DOI] [PubMed] [Google Scholar]
- 6.Nelson JA, Strauss L, Skowronski M, Ciufo R, Novak R, McFadden ER., Jr Effect of long-term salmeterol treatment on exercise-induced asthma. N Engl J Med. 1998;339:141–146. doi: 10.1056/NEJM199807163390301. [DOI] [PubMed] [Google Scholar]
- 7.Haney S, Hancox RJ. Recovery from bronchoconstriction and bronchodilator tolerance. Clin Rev Allergy Immunol. 2006;31:181–196. doi: 10.1385/CRIAI:31:2:181. [DOI] [PubMed] [Google Scholar]
- 8.Liggett SB. Polymorphisms of the beta2-adrenergic receptor and asthma. Am J Respir Crit Care Med. 1997;156:S156–S162. doi: 10.1164/ajrccm.156.4.12tac-15. [DOI] [PubMed] [Google Scholar]
- 9.Israel E, Drazen JM, Liggett SB, Boushey HA, Cherniack RM, Chinchilli VM, Cooper DM, Fahy JV, Fish JE, Ford JG, et al. The effect of polymorphisms of the beta(2)-adrenergic receptor on the response to regular use of albuterol in asthma. Am J Respir Crit Care Med. 2000;162:75–80. doi: 10.1164/ajrccm.162.1.9907092. [DOI] [PubMed] [Google Scholar]
- 10.Israel E, Chinchilli VM, Ford JG, Boushey HA, Cherniack R, Craig TJ, Deykin A, Fagan JK, Fahy JV, Fish J, et al. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet. 2004;364:1505–1512. doi: 10.1016/S0140-6736(04)17273-5. [DOI] [PubMed] [Google Scholar]
- 11.Wechsler ME, Lehman E, Lazarus SC, Lemanske RF, Jr, Boushey HA, Deykin A, Fahy JV, Sorkness CA, Chinchilli VM, Craig TJ, et al. beta-Adrenergic receptor polymorphisms and response to salmeterol. Am J Respir Crit Care Med. 2006;173:519–526. doi: 10.1164/rccm.200509-1519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DK, Currie GP, Hall IP, Lima JJ, Lipworth BJ. The arginine-16 beta2-adrenoceptor polymorphism predisposes to bronchoprotective subsensitivity in patients treated with formoterol and salmeterol. Br J Clin Pharmacol. 2004;57:68–75. doi: 10.1046/j.1365-2125.2003.01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snyder EM, Beck KC, Dietz NM, Joyner MJ, Turner ST, Johnson BD. Influence of beta2-adrenergic receptor genotype on airway function during exercise in healthy adults. Chest. 2006;129:762–770. doi: 10.1378/chest.129.3.762. [DOI] [PubMed] [Google Scholar]
- 14.Nijkamp FP, Henricks PA. Receptors in airway disease. Beta-adrenoceptors in lung inflammation. Am Rev Respir Dis. 1990;141:S145–S150. doi: 10.1164/ajrccm/141.3_Pt_2.S145. [DOI] [PubMed] [Google Scholar]
- 15.Brusasco V, Crapo R, Viegi G. Coming together: the ATS/ERS consensus on clinical pulmonary function testing. Eur Respir J. 2005;26:1–2. doi: 10.1183/09031936.05.00034205. [DOI] [PubMed] [Google Scholar]
- 16.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 17.American Thoracic Society, European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 18.Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–907. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 19.Bonini M, Di Mambro C, Calderon MA, Compalati E, Schünemann H, Durham S, Canonica GW. Beta2-agonists for exercise-induced asthma. Cochrane Database Syst Rev. 2013;10:CD003564. doi: 10.1002/14651858.CD003564.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Summerhill E, Leavitt SA, Gidley H, Parry R, Solway J, Ober C. beta(2)-adrenergic receptor Arg16/Arg16 genotype is associated with reduced lung function, but not with asthma, in the Hutterites. Am J Respir Crit Care Med. 2000;162:599–602. doi: 10.1164/ajrccm.162.2.9910108. [DOI] [PubMed] [Google Scholar]
- 21.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin AC, Plummer AL, Taylor DR. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanazawa H, Hirata K, Yoshikawa J. Role of endogenous nitric oxide in exercise-induced airway narrowing in patients with bronchial asthma. J Allergy Clin Immunol. 2000;106:1081–1087. doi: 10.1067/mai.2000.110803. [DOI] [PubMed] [Google Scholar]
- 23.ElHalawani SM, Ly NT, Mahon RT, Amundson DE. Exhaled nitric oxide as a predictor of exercise-induced bronchoconstriction. Chest. 2003;124:639–643. doi: 10.1378/chest.124.2.639. [DOI] [PubMed] [Google Scholar]
- 24.Smith AD, Cowan JO, Brassett KP, Filsell S, McLachlan C, Monti-Sheehan G, Peter Herbison G, Robin Taylor D. Exhaled nitric oxide: a predictor of steroid response. Am J Respir Crit Care Med. 2005;172:453–459. doi: 10.1164/rccm.200411-1498OC. [DOI] [PubMed] [Google Scholar]
- 25.Kanazawa H, Shiraishi S, Okamoto T, Hirata K, Yoshikawa J. Inhibition of bronchoprotective effects of beta2-adrenoceptor agonists by peroxynitrite in guinea pig airways. Am J Respir Crit Care Med. 1999;159:1272–1276. doi: 10.1164/ajrccm.159.4.9808009. [DOI] [PubMed] [Google Scholar]
- 26.Fitch KD. An overview of asthma and airway hyper-responsiveness in Olympic athletes. Br J Sports Med. 2012;46:413–416. doi: 10.1136/bjsports-2011-090814. [DOI] [PubMed] [Google Scholar]