Abstract
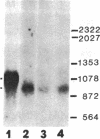
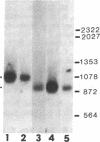
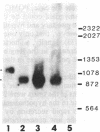
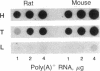
Adrenocorticotropin (ACTH), beta-endorphin, and the melanocyte-stimulating hormones (MSHs), which are products of a common precursor, pro-opiomelanocortin (POMC), are present in a variety of tissues other than pituitary. The recent detection of immunoreactive POMC-derived peptides in the male reproductive tract raised the possibility that these hormones might regulate reproductive function. To determine whether the low concentrations of POMC-derived peptides in the male reproductive tract are synthesized locally and are not contaminants from blood, we have demonstrated POMC-like gene expression in both testis and epididymis. The identification of cells in testis capable of synthesizing POMC mRNA was established by showing the presence of this mRNA in mouse Leydig cell lines (TM3 and I10A). The hybridizing species of POMC-like mRNA in the testis, epididymis, and Leydig cell lines (TM3 and I10A) were approximately 150 bases shorter than those in the pituitary or hypothalamus but were similar in size to that in the amygdaloid nucleus of rat brain. The concentration of POMC-like mRNA in the testis is almost as high as that in the hypothalamus. This finding is quite unexpected because the concentrations of POMC-derived peptides in the testis were 2-3 orders of magnitude lower than those in the hypothalamus. The demonstration of a POMC-like gene expression in male reproductive tissues suggests that POMC-derived peptides are synthesized in Leydig cells and epididymis. These observations are consistent with the postulate that POMC-derived peptides may exert paracrine and/or autocrine effects in these organs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramowitz J., Campbell A. R. Enkephalin-mediated inhibition of forskolin-stimulated rabbit luteal adenylyl cyclase activity. Biochem Biophys Res Commun. 1983 Oct 31;116(2):574–580. doi: 10.1016/0006-291x(83)90562-4. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni J. F., Watkins W. B., Yen S. S. beta-Endorphin in the human pancreas. J Clin Endocrinol Metab. 1979 Oct;49(4):649–651. doi: 10.1210/jcem-49-4-649. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Nunberg J. H., Kaufman R. J., Erlich H. A., Schimke R. T., Cohen S. N. Phenotypic expression in E. coli of a DNA sequence coding for mouse dihydrofolate reductase. Nature. 1978 Oct 19;275(5681):617–624. doi: 10.1038/275617a0. [DOI] [PubMed] [Google Scholar]
- Chen C. L., Dionne F. T., Roberts J. L. Regulation of the pro-opiomelanocortin mRNA levels in rat pituitary by dopaminergic compounds. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2211–2215. doi: 10.1073/pnas.80.8.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civelli O., Birnberg N., Herbert E. Detection and quantitation of pro-opiomelanocortin mRNA in pituitary and brain tissues from different species. J Biol Chem. 1982 Jun 25;257(12):6783–6787. [PubMed] [Google Scholar]
- Deshpande A. K., Chatterjee B., Roy A. K. Translation and stability of rat liver messenger RNA for alpha 2 mu-globulin in Xenopus oocyte. The role of terminal poly(A). J Biol Chem. 1979 Sep 25;254(18):8937–8942. [PubMed] [Google Scholar]
- Drouin J., Goodman H. M. Most of the coding region of rat ACTH beta--LPH precursor gene lacks intervening sequences. Nature. 1980 Dec 11;288(5791):610–613. doi: 10.1038/288610a0. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E. Structure and biosynthesis of pro-adrenocorticotropin/endorphin and related peptides. Endocr Rev. 1980 Winter;1(1):1–27. doi: 10.1210/edrv-1-1-1. [DOI] [PubMed] [Google Scholar]
- Evans C. J., Erdelyi E., Weber E., Barchas J. D. Identification of pro-opiomelanocortin-derived peptides in the human adrenal medulla. Science. 1983 Sep 2;221(4614):957–960. doi: 10.1126/science.6308766. [DOI] [PubMed] [Google Scholar]
- Gerendai I., Nemeskéri A., Csernus V. Naloxone has a local effect on the testis of immature rats. Andrologia. 1983 Jul-Aug;15(4):398–403. doi: 10.1111/j.1439-0272.1983.tb00160.x. [DOI] [PubMed] [Google Scholar]
- Jacobson A., Favreau M. Possible involvement of poly(A) in protein synthesis. Nucleic Acids Res. 1983 Sep 24;11(18):6353–6368. doi: 10.1093/nar/11.18.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger D. T. Brain peptides: what, where, and why? Science. 1983 Dec 2;222(4627):975–985. doi: 10.1126/science.6139875. [DOI] [PubMed] [Google Scholar]
- Krieger D. T., Liotta A. S., Brownstein M. J., Zimmerman E. A. ACTH, beta-lipotropin, and related peptides in brain, pituitary, and blood. Recent Prog Horm Res. 1980;36:277–344. doi: 10.1016/b978-0-12-571136-4.50015-2. [DOI] [PubMed] [Google Scholar]
- Larsson L. I. Adrenocorticotropin-like and alpha-melanotropin-like peptides in a subpopulation of human gastrin cell granules: bioassay, immunoassay, and immunocytochemical evidence. Proc Natl Acad Sci U S A. 1981 May;78(5):2990–2994. doi: 10.1073/pnas.78.5.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroith D., Liotta A. S., Roth J., Shiloach J., Lewis M. E., Pert C. B., Krieger D. T. Corticotropin and beta-endorphin-like materials are native to unicellular organisms. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2086–2090. doi: 10.1073/pnas.79.6.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim A. T., Lolait S., Barlow J. W., O W. S., Zois I., Toh B. H., Funder J. W. Immunoreactive beta-endorphin in sheep ovary. Nature. 1983 Jun 23;303(5919):709–711. doi: 10.1038/303709a0. [DOI] [PubMed] [Google Scholar]
- Liotta A. S., Gildersleeve D., Brownstein M. J., Krieger D. T. Biosynthesis in vitro of immunoreactive 31,000-dalton corticotropin/beta-endorphin-like material by bovine hypothalamus. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1448–1452. doi: 10.1073/pnas.76.3.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta A. S., Houghten R., Krieger D. T. Identification of a beta-endorphin-like peptide in cultured human placental cells. Nature. 1982 Feb 18;295(5850):593–595. doi: 10.1038/295593a0. [DOI] [PubMed] [Google Scholar]
- Liotta A. S., Krieger D. T. In vitro biosynthesis and comparative posttranslational processing of immunoreactive precursor corticotropin/beta-endorphin by human placental and pituitary cells. Endocrinology. 1980 May;106(5):1504–1511. doi: 10.1210/endo-106-5-1504. [DOI] [PubMed] [Google Scholar]
- Liotta A. S., Loudes C., McKelvy J. F., Krieger D. T. Biosynthesis of precursor corticotropin/endorphin-, corticotropin-, alpha-melanotropin-, beta-lipotropin-, and beta-endorphin-like material by cultured neonatal rat hypothalamic neurons. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1880–1884. doi: 10.1073/pnas.77.4.1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margioris A. N., Liotta A. S., Vaudry H., Bardin C. W., Krieger D. T. Characterization of immunoreactive proopiomelanocortin-related peptides in rat testes. Endocrinology. 1983 Aug;113(2):663–671. doi: 10.1210/endo-113-2-663. [DOI] [PubMed] [Google Scholar]
- Mather J. P. Establishment and characterization of two distinct mouse testicular epithelial cell lines. Biol Reprod. 1980 Aug;23(1):243–252. doi: 10.1095/biolreprod23.1.243. [DOI] [PubMed] [Google Scholar]
- Orwoll E. S., Kendall J. W. Beta-endorphin and adrenocorticotropin in extrapituitary sites: gastrointestinal tract. Endocrinology. 1980 Aug;107(2):438–442. doi: 10.1210/endo-107-2-438. [DOI] [PubMed] [Google Scholar]
- Payvar F., Schimke R. T. Methylmercury hydroxide enhancement of translation and transcription of ovalbumin and conalbumin mRNA's. J Biol Chem. 1979 Aug 25;254(16):7636–7642. [PubMed] [Google Scholar]
- Roberts J. L., Chen C. L., Eberwine J. H., Evinger M. J., Gee C., Herbert E., Schachter B. S. Glucocorticoid regulation of proopiomelanocortin gene expression in rodent pituitary. Recent Prog Horm Res. 1982;38:227–256. doi: 10.1016/b978-0-12-571138-8.50011-1. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. G., Mermod J. J., Amara S. G., Swanson L. W., Sawchenko P. E., Rivier J., Vale W. W., Evans R. M. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature. 1983 Jul 14;304(5922):129–135. doi: 10.1038/304129a0. [DOI] [PubMed] [Google Scholar]
- Shaha C., Liotta A. S., Krieger D. T., Bardin C. W. The ontogeny of immunoreactive beta-endorphin in fetal, neonatal, and pubertal testes from mouse and hamster. Endocrinology. 1984 May;114(5):1584–1591. doi: 10.1210/endo-114-5-1584. [DOI] [PubMed] [Google Scholar]
- Sharp B., Pekary A. E., Meyer N. V., Hershman J. M. beta-Endorphin in male rat reproductive organs. Biochem Biophys Res Commun. 1980 Jul 31;95(2):618–623. doi: 10.1016/0006-291x(80)90830-x. [DOI] [PubMed] [Google Scholar]
- Sharp B., Pekary A. E. beta-Endorphin 61-91 and other beta-endorphin-immunoreactive peptides in human semen. J Clin Endocrinol Metab. 1981 Mar;52(3):586–588. doi: 10.1210/jcem-52-3-586. [DOI] [PubMed] [Google Scholar]
- Shin S. I. Studies on interstitial cells in tissue culture: steroid biosynthesis in monolayers of mouse testicular interstitial cells. Endocrinology. 1967 Sep;81(3):440–448. doi: 10.1210/endo-81-3-440. [DOI] [PubMed] [Google Scholar]
- Shu-Dong T., Phillips D. M., Halmi N., Krieger D., Bardin C. W. Beta-endorphin is present in the male reproductive tract of five species. Biol Reprod. 1982 Oct;27(3):755–764. doi: 10.1095/biolreprod27.3.755. [DOI] [PubMed] [Google Scholar]
- Smith E. M., Blalock J. E. Human lymphocyte production of corticotropin and endorphin-like substances: association with leukocyte interferon. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7530–7534. doi: 10.1073/pnas.78.12.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. M., Meyer W. J., Blalock J. E. Virus-induced corticosterone in hypophysectomized mice: a possible lymphoid adrenal axis. Science. 1982 Dec 24;218(4579):1311–1312. doi: 10.1126/science.6183748. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsong S. D., Phillips D., Halmi N., Liotta A. S., Margioris A., Bardin C. W., Krieger D. T. ACTH and beta-endorphin-related peptides are present in multiple sites in the reproductive tract of the male rat. Endocrinology. 1982 Jun;110(6):2204–2206. doi: 10.1210/endo-110-6-2204. [DOI] [PubMed] [Google Scholar]
- Vournakis J. N., Gelinas R. E., Kafatos F. C. Short polyadenylic acid sequences in insect chorion messenger RNA. Cell. 1974 Nov;3(3):265–273. doi: 10.1016/0092-8674(74)90141-x. [DOI] [PubMed] [Google Scholar]
- Young R. A., Hagenbüchle O., Schibler U. A single mouse alpha-amylase gene specifies two different tissue-specific mRNAs. Cell. 1981 Feb;23(2):451–458. doi: 10.1016/0092-8674(81)90140-9. [DOI] [PubMed] [Google Scholar]