Abstract
Pleomorphic adenoma is the most common benign neoplasm of the salivary gland. Few studies are currently available on pleomorphic adenoma cell apoptosis. The aim of this study was to investigate the effect of cyclopamine induction apoptosis in human salivary pleomorphic adenoma (HSPA) cells and the impact on Gli2 and Bcl2 mRNA levels. Cells were quantified and cell morphology was visualized under microscope. Flow cytometry was used to detect the apoptotic rate. Cyclopamine is considered an efficient blocker of the hedgehog (Hh) signaling pathway. Following treatment with 10 μmol/l cyclopamine for 48 h, the number of cells were reduced, and nuclear pycnosis or fragmentation, as well as chromatospherite disfiguration apoptotic morphology were observed under microscope. One-way ANOVA test results revealed a significantly greater decrease (P<0.01) of Gli2 and Bcl2 mRNA levels in the cyclopamine-treated group as compared to the blank control group and dimethyl sulfoxide (DMSO)-treated group. Following treatment with 10 μmol/l cyclopamine for 24 h, the apoptotic rate of the cyclopamine-treated group was significantly higher than that of the blank control and DMSO-treated group (P<0.01). Findings of this study showed that cyclopamine affected the mechanism of HSPA cell apoptosis, which may be associated with the downregulation of Gli2 and Bcl2 mRNA expression levels and the activation of the mitochondrial apoptotic pathways.
Keywords: pleomorphic adenoma cell, apoptosis, cyclopamine, Gli2, Bcl2
Introduction
Pleomorphic adenoma (PA) is the most common benign neoplasm of the salivary gland. It occurs most often between the ages of 30 and 60 years and is detected more commonly in females than in males. Although the tumor is usually benign, recurrence may occur when it is inadequately excised. Moreover, 2–17% of the tumors can progress to malignancy, resulting in carcinoma ex-PA, an aggressive malignancy that may metastasize and result in death. The biological behavior of the neoplasm is thought to be associated primarily with genetic alterations in the tumor cells themselves. Differential gene expression was validated for the upregulated gene, Gli2, using real-time PCR and the results were consistent with those of the cDNA microarray analysis thus verifying the credibility of the microarray data (1). Gli2 is a nuclear transcription factor of the Hedgehog (Hh) signaling pathway, a key regulator in embryogenesis where it affects processes such as cell proliferation, differentiation and tissue patterning. In adults it was involved in the maintenance of stem cells and in tissue repair and regeneration. However, this pathway was also an important in various types of human cancer where it promoted growth and enabled the proliferation of tumor stem cells. Results of a recent clinical study (2) have shown that Hh signaling is the basis of an important new class of therapeutic agents with far-reaching implications in oncology. In the present study, the role of cyclopamine as a Hh signaling pathway inhibitor in PA cells and in the detection of PA cell apoptosis was investigated. Additionally, cyclopamine was utilized to detect expression of apoptotic gene Bcl2. To gain a better understanding of the human salivary PA of the molecular pathogenesis, we also investigated the mechanism of the Hh signaling pathway in human PA-induced apoptosis. Results demonstrated that our data may provide clues for the identification of new targets for the diagnosis and therapy of PA.
Materials and methods
Materials
DMEM-F12 joint medium supplemented with 10% fetal bovine serum (Invitrogen Life Technologies, Carlsbad, CA, USA), 20 ng/ml epidermal growth factor (ProSpec, Rehovot, Israel), 5 μg/ml insulin (Sigma, St. Louis, MO, USA), 0.4 μg/ml hydrocortisone (ProSpec), penicillin (500 U/ml) and streptomycin (500 U/ml), and 0.25% trypsin (Sigma) were utilized. Cyclopamine and dimethyl sulfoxide (DMSO) were purchased from Sigma. Reverse transcriptase-polymerase chain reaction (RT-PCR) and SYBR-Green kits were purchased from Takara Bio, Inc. (Tokyo, Japan). Cyclopamine (1 mg at a purity of ≥98%) was dissolved in 200 μl of DMSO placed in a 60°C water bath box for ∼1 min.
PA cells were cultured in human salivary pleomorphic adenoma (HSPA). The complete medium was incubated at 37°C 5% CO2 every 3 to 4 days and passaged at a dilution of 1:2. Logarithmic phase cells were used in the experiment.
Cells with a density of 5×105/ml were grown in a petri dish with a diameter of 10 cm. After 24 h in serum-free medium, cells were synchronized. The medium in the petri dish was replaced with complete medium (8 ml). Cells were treated with 10 μmol/l cyclopamine (3) or 0.08% DMSO for 48 h. The blank control group was not treated with the drug. Group cell numbers and morphological changes were observed under an inverted microscope.
RNA extraction
Cells (2×105) were cultivated in 6-well plates. Cells were synchronized initially in 3 ml complete medium and 24 h in serum-free medium. The medium was replaced with complete medium and cyclopamine was added at a final concentration of 10 μmol/l (3). The DMSO group was treated and DMSO was adjusted to a final concentration of 0.08%. Total RNA was extracted after 48 h using TRIzol reagent. Electrophoresis was used to identify the typical RNA bands. Since the three bands identified were uniform and concentrated, there was no apparent degradation for RNA. RNA samples from the HSPA cells were extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Total RNA was then reverse-transcribed into cDNA according to the instructions of the reverse transcription kit (Takara).
GAPDH, Bcl2 and Gli2 primers were synthesized by Shanghai Boshang (Shanghai, China). Real-time PCR was performed using the ABI Prism®7300 real-time PCR system according to the manufacturer’s instructions. Primer sequences were: GAPDH, upstream: 5′-AAGGTGAAGGTCGGAGT CAAC-3′ and downstream: 5′-GGGGTCATTGATGGCAAC AATA-3′, with a product length of 102 bp; Bcl2, upstream: 5′-AAGATTGATGGGATCGTTGC-3′ and downstream: 5′-GCGGAACACTTGATTCTGGT-3′, with a product length of 229 bp; Gli2, upstream: 5′-CATGGAGCACTACCTCC GTTC-3′ and downstream: 5′-CGACGGTCATCTGGTGG TAAT-3′, with a product length of 172 bp. The total reaction volume was 20 μl (1 μl cDNA, 10 μl SYBR Premix Ex Taq, 1 μl upstream primer, 1 μl downstream primer and 7 μl ddH2O). The reaction conditions were: activation at 95°C for 30 sec, 40 cycles of denaturation at 95°C for 5 sec, primer annealing and extension at 60°C for 31 sec and further extension at 95°C. Melting curve analysis of the samples was routinely performed to ascertain that only the expected products had been generated. mRNA expression levels of target genes were normalized to the expression of GAPDH and calculated using the 2-ΔΔCt method.
Logarithmic measurements
Logarithmic phase HSPA cells were inoculated at a density of 2×105 cells/well in 6-well plates and cultivated overnight. The cells were synchronized after 24 h in serum-free medium. The culture medium was removed and the experimental group was treated with 10 μmol/l cyclopamine. The DMSO-treated group was treated with 0.08% DMSO. The blank control group was not treated with the drug. After 24 h, the single cell suspension was digested with 0.25% trypsin. Annexin-V-FITC (annexin V) and pyridine iodide double staining were carried out according to the manufacturer’s instructions. Cell apoptosis was detected by flow cytometry and the experimental results were performed in triplicate.
Statistical analysis
Data were presented as the mean ± standard deviation and analyzed using SAS 9.13 software. P-values were calculated, with P<0.01 being statistically significant. Groups were compared using one-way analysis of variance at an inspection level of α=0.01.
Results
Morphological changes of HSPA cells
Unlike the blank control and DMSO-treated groups, the addition of 10 μmol/l of cyclopamine to the HSPA cells in three-dimensional sense of loss, resulted in a decrease in cell size. The cells formed a circle, while the cell gap increased, as observed under an inverted microscope. The morphology of HSPA cells altered significantly following the addition of cyclopamine (Fig. 1).
Figure 1.
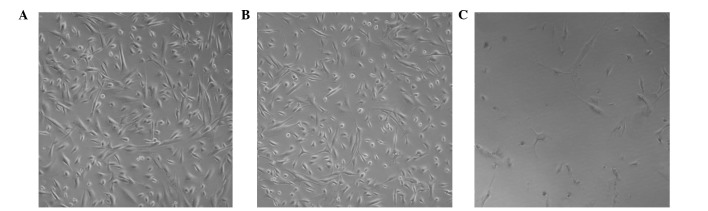
Morphological changes of human salivary pleomorphic adenoma cells observed under an inverted microscope (magnification, ×200): (A) Blank control group; (B) dimethyl sulfoxide-treated group (0.08% DMSO, 48 h); (C) Cyclopamine-treated group (10 μmol/l, 48 h).
Effects of cyclopamine on mRNA expression of Gli2 and Bcl2
The amount of mRNA of the target genes relative to GAPDH was calculated using the formula 2-ΔΔCt, while the change in fold expression was calculated relative to the blank control group. The results showed that compared with the blank control and DMSO-treated groups, Gli2 and Bcl2 mRNA expression levels of the cyclopamine-treated group were significantly lower. No significant differences were observed in the DMSO-treated and blank control groups (P>0.05) (Fig. 2).
Figure 2.
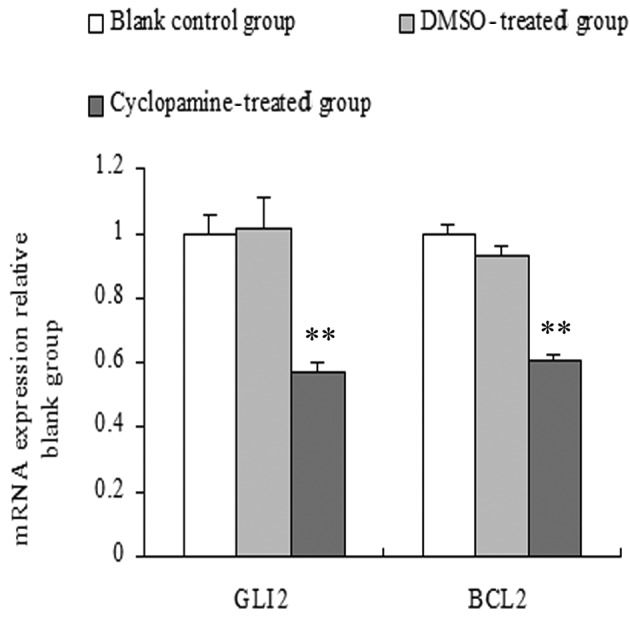
Effects of cyclopamine on the mRNA expression of Gli2 and Bcl2 in human salivary pleomorphic adenoma cells as compared with the blank control and dimethyl sulfoxide-treated groups, **P<0.01.
Effect of cyclopamine on HSPA cells
The apoptotic rate of the blank control group (apoptosis rate = early apoptosis + late apoptosis rate) was (7.10±1.23)%, the DMSO-treated group was (8.18±1.98)%, and the 10 μmol/l cyclopamine-treated group was (20.45±3.27)%. The apoptotic rate of the cyclopamine group was significantly higher compared with that of the blank control and DMSO-treated groups (F=40.94, P<0.01). No significant differences were detected in the DMSO-treated and blank control groups (P>0.05) (Fig. 3).
Figure 3.
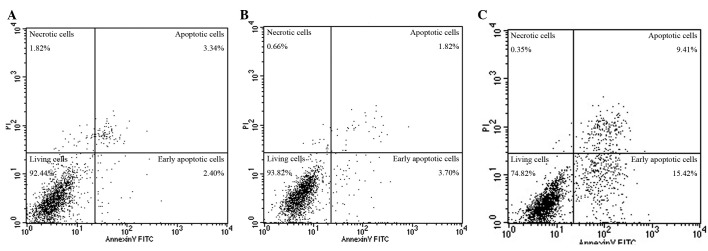
Effect of cyclopamine on apoptosis of human salivary pleomorphic adenoma (HSPA) cells: (A) Blank control group (apoptotic rate, 5.74%), (B) dimethyl sulfoxide-treated group (apoptotic rate, 5.52%), (C) cyclopamine-treated group (apoptosis rate 24.83%).
Discussion
Parotid PA is a borderline tumor that is treated mainly by surgical resection. The tumor capsule is unevenly thick and incomplete, thus tumor cells usually infiltrate both within and outside the capsule, resulting in poor adhesion between the tumor and its capsule. Surgical resection also has numerous defective impacts including facial scarring as well as complications such as Frey’s syndrome, earlobe numbness, recurrence and malignant sequelae. Consequently, studies have focused on basic investigation into PA. The Hh signaling pathway is considered a key regulator in embryogenesis where it regulates processes such as cell proliferation, differentiation and tissue patterning (4,5). In adults it is involved in the maintenance of stem cells and in tissue repair and regeneration. It is also involved in several types of human cancer, promoting the growth and proliferation of tumor stem cells. Effector molecules involved in tumor cell proliferation, such as BMP and cyclin, have been proven to be target genes of the Hh pathway or downstream molecules (6). Crosstalk exists between the Hh signaling pathway and other signaling pathways, including the Notch and Wnt, Ras-of Erk pathways (6–8).
Cyclopamine is the most potent specific inhibitor of the Hh signaling pathway. It is able to downregulate the activity of this pathway, thereby inhibiting the excessive activation of tumor cell proliferation.
Hh signaling pathway comprises three Hh ligands [Sonic Hh (Shh), Indian Hh (Ihh) and Desert Hh (Dhh)], two transmembrane protein receptors [Patched (Ptch) and Smoothened (Smo)], three nuclear transcription factors (Gli1, -2 and -3) and downstream target genes (Ptch, Gli, Wnt, EGF and cyclins). Gli2, which has a length of 3,678 bp and is localized at 2q14, is a zinc finger transcription factor of the Hh signaling transduction pathway. Recent studies have confirmed (1) that the Gli2 gene mRNA expression in PA was significantly higher compared with the normal tissue adjacent to the tumor, suggesting that Gli2 is capable of inducing the proliferation of PA cells. Prostate cancer cells using Gli2-specific small hairpin RNA knock down Gli2 in these cells, resulting in the downregulation of the Hh signaling pathway and inhibition of the growth of tumor tissue xenografts in vivo (9). Conversely, the ectopic expression of Gli2 in non-tumorigenic prostate epithelial cells resulted in accelerated cell cycle progression, particularly transition through G2-M and increased proliferation (9).
Apoptotic signaling is activated via the death receptor and mitochondrial pathways. The death receptor pathway is triggered through ligand activation of the abnormal cell surface death receptors, including CD95/Fas, tumor necrosis factor receptor and death receptor. Ligand binding with the receptor, combined with the Fas-associated death domain protein generates a death-inducing signaling complex. Additionally, activating caspase cascade is likely to cause irreversible damage in cells. The mitochondrial pathway is mainly triggered by oxidative stress, DNA damage, cancer chemotherapeutic drugs and ionizing radiation, which can increase the permeability of the mitochondrial membrane, and promote the release of cytochrome C. Cytochrome C can be combined with apoptotic protease activation factor-1 and activate caspase 9 precursor. Additionally, the formation of apoptotic bodies and activation of caspase downstream effectors is able to induce DNA fragmentation and cell death (10–12).
Bcl2 family proteins, divided into anti-apoptotic (Bcl2, Bcl-G) and pro-apoptotic (Bax, Bad, Bid) proteins, regulate the activity of the mitochondrial pathway. Bid and Bax proteins promote the mitochondrial release of Cyt-C and induce apoptosis. However, Bcl2 inhibited mitochondrial-releasing cytochrome C and apoptosis. In this study, the Hh pathway inhibitor cyclopamine decreased activity of the Hh pathway and downregulated Gli2 and Bcl2 mRNA expression levels. We hypothesized that the cyclopamine-inducing apoptosis mechanism in HSPA cells may be associated with the process of the decreased expression of Bcl2 following the downregulation of Gli2. As the decreased expression of Bcl2 activates the mitochondrial pathway of apoptosis, it is likely to increase the release of mitochondrial cytochrome C and activate the downstream caspase cascade, leading to irreversible cell damage. Previous studies have shown that Bcl2 is a Gli target gene of the Hh signaling pathway (13–15). Gli is able to regulate the expression of Bcl2 (16). In a study on basal cell carcinoma, Bigelow et al (15) observed that the Bcl2 promoter has seven potential Gli anchor points. Gli proteins regulate Bcl2 activity through transcriptional regulation of the Bcl2 promoter. Bar et al (14) found that in medulloblastoma samples, Gli1 was clearly correlated with Bcl2 mRNA levels. Improving the transfection of Gli1 mRNA expression yielded a corresponding increase in Bcl2 mRNA. Thus, when cyclopamine blocks Hh signaling pathway activity to decrease Gli1 mRNA expression, a corresponding decrease in Bcl2 mRNA occurs, thereby promoting apoptosis of tumor cells. The Bcl2 gene has two promoters, E1P1+ and E2P2. The E1P1+ sequence contains three nucleic acid sequences (CGCCACCCA, GACCACCAA and GCACACCCA) which are highly homologous with the Gli family of protein-binding elements. Restructuring of the Gli1 protein can be combined with the Bcl2 promoter fragment containing the GACCACCAA sequence, suggesting that Bcl2 is regulated by Gli1 target genes. Similar events also occur in the Gli2 gene. Activation of Bcl2 by Gli2 is stronger compared with that of Gli1, whose gene expression can be strengthened >10-fold (13). Hepatocellular carcinoma studies have shown that cyclopamine inhibits Hh signaling which highly expresses the Gli signaling factor. By downregulating the expression of Bcl2, Hh signaling induces apoptosis of hepatoma cells (17). Studies on pancreatic cancer (18), prostate cancer (19), colon cancer (20), gastric cancer (21), medulloblastoma (22) and basal cell carcinoma (15) obtained similar results, thus Hh signaling occurs via the regulation of Bcl2 gene to manipulate tumor cell apoptosis.
Findings of recent studies suggest that cyclopamine is a new method for the treatment of tumors by inducing apoptosis of malignant cells (23). The Hh signaling pathway constitutes an important target. Findings of this study have demonstrated that inhibition of transduction of the Hh signaling pathway results in an enhanced HSPA cell apoptotic rate, obvious morphological changes of apoptosis and decrease of the anti-apoptotic factor Bcl2 mRNA expression. These findings are crucial in the pathogenesis of PAs, as well as the promotion of tumor cell apoptosis and drug targets. Therefore, cyclopamine is potentially of great significance to the prevention, treatment and prognosis of PA.
Acknowledgments
This study was supported by grant nos. 08JC1412900 and 10DZ1951300 from the Science and Technology Commission of Shanghai Municipality and grant no. S30206 from the Shanghai Leading Academic Discipline Project.
References
- 1.Song M, Xiao C, Wang T, et al. Study of the differentially expressed genes in pleomorphic adenoma using cDNA micro-arrays. Pathol Oncol Res. 2011;17:765–769. doi: 10.1007/s12253-011-9384-9. [DOI] [PubMed] [Google Scholar]
- 2.Kelleher FC, Cain JE, Healy JM, et al. Prevailing importance of the hedgehog signaling pathway and the potenial for treatment advancement in sarcoma. Pharmacol Ther. 2012;136:153–168. doi: 10.1016/j.pharmthera.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Mimeault M, Moore E, Moniaux N, et al. Cytotoxic effects induced by a combination of cyclopamine and gefitinib, the selective hedgehog and epidermal growth factor receptor signaling inhibitors, in prostate cancer cells. Int J Cancer. 2006;118:1022–1031. doi: 10.1002/ijc.21440. [DOI] [PubMed] [Google Scholar]
- 4.Pasca di Magliano M, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat Rev Cancer. 2003;3:903–911. doi: 10.1038/nrc1229. [DOI] [PubMed] [Google Scholar]
- 5.Marini KD, Payne BJ, Watkins DN, Martelotto LG. Mechanisms of Hedgehog signaling in cancer. Growth Factors. 2011;29:221–234. doi: 10.3109/08977194.2011.610756. [DOI] [PubMed] [Google Scholar]
- 6.Paiva KB, Silva-Valenzuela MD, Massironi SM, et al. Differential Shh, Bmp and Wnt gene expressions during craniofacial development in mice. Acta Histochem. 2010;112:508–517. doi: 10.1016/j.acthis.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Shi I, Hashemi Sadraei N, Duan ZH, Shi T. Aberrant signaling pathways in squamous cell lung carcinoma. Cancer Inform. 2011;10:273–285. doi: 10.4137/CIN.S8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Min TH, Kriebel M, Hou S, Pera EM. The dual regulator Sufu integrates Hedgehog and Wnt signals in the early Xenopus embryo. Dev Biol. 2011;358:262–276. doi: 10.1016/j.ydbio.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 9.Thiyagarajan S, Bhatia N, Reagan-Shaw S, et al. Role of GLI2 transcription factor in growth and tumorigenicity of prostate cells. Cancer Res. 2007;67:10642–10646. doi: 10.1158/0008-5472.CAN-07-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iannolo G, Conticello C, Memeo L, De Maria R. Apoptosis in normal and cancer stem cells. Crit Rev Oncol Hematol. 2008;66:42–51. doi: 10.1016/j.critrevonc.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Qiao L, Wong BC. Targeting apoptosis as an approach for gastrointestinal cancer therapy. Drug Resist Updat. 2009;12:55–64. doi: 10.1016/j.drup.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Chen XL, Cao LQ, She MR, et al. Gli-1 siRNA induced apoptosis in Huh7 cells. World J Gastroenterol. 2008;14:582–589. doi: 10.3748/wjg.14.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regl G, Kasper M, Schnidar H, et al. Activation of the BCL2 promoter in response to Hedgehog/GLI signal transduction is predominantly mediated by GLI2. Cancer Res. 2004;64:7724–7731. doi: 10.1158/0008-5472.CAN-04-1085. [DOI] [PubMed] [Google Scholar]
- 14.Bar EE, Chaudhry A, Farah MH, Eberhart CG. Hedgehog signaling promotes medulloblastoma survival via Bc/II. Am J Pathol. 2007;170:347–355. doi: 10.2353/ajpath.2007.060066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bigelow RL, Chari NS, Unden AB, et al. Transcriptional regulation of Bcl-2 mediated by the sonic hedgehog signaling pathway through gli-1. J Biol Chem. 2004;279:1197–1205. doi: 10.1074/jbc.M310589200. [DOI] [PubMed] [Google Scholar]
- 16.Katoh Y, Katoh M. Hedgehog target genes: mechanisms of carcinogenesis induced by aberrant hedgehog signaling activation. Curr Mol Med. 2009;9:873–886. doi: 10.2174/156652409789105570. [DOI] [PubMed] [Google Scholar]
- 17.Chen XL, Cheng QY, She MR, et al. Expression of sonic hedgehog signaling components in hepatocellular carcinoma and cyclopamine-induced apoptosis through Bcl-2 downregulation in vitro. Arch Med Res. 2010;41:315–323. doi: 10.1016/j.arcmed.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Mo W, Xu X, Xu L, et al. Resveratrol inhibits proliferation and induces apoptosis through the hedgehog signaling pathway in pancreatic cancer cell. Pancreatology. 2012;11:601–609. doi: 10.1159/000333542. [DOI] [PubMed] [Google Scholar]
- 19.Farooqi AA, Mukhtar S, Riaz AM, et al. Wnt and SHH in prostate cancer: trouble mongers occupy the TRAIL towards apoptosis. Cell Prolif. 2011;44:508–515. doi: 10.1111/j.1365-2184.2011.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazumdar T, DeVecchio J, Shi T, et al. Hedgehog signaling drives cellular survival in human colon carcinoma cells. Cancer Res. 2011;71:1092–1102. doi: 10.1158/0008-5472.CAN-10-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han ME, Lee YS, Baek SY, et al. Hedgehog signaling regulates the survival of gastric cancer cells by regulating the expression of Bcl-2. Int J Mol Sci. 2009;10:3033–3043. doi: 10.3390/ijms10073033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCall TD, Pedone CA, Fults DW. Apoptosis suppression by somatic cell transfer of Bcl-2 promotes Sonic hedgehog-dependent medulloblastoma formation in mice. Cancer Res. 2007;67:5179–5185. doi: 10.1158/0008-5472.CAN-06-4177. [DOI] [PubMed] [Google Scholar]
- 23.Kurita S, Mott JL, Cazanava SC, et al. Hedgehog inhibition promotes a switch from Type II to Type I cell death receptor signaling in cancer cells. PLoS One. 2011;6:e18330. doi: 10.1371/journal.pone.0018330. [DOI] [PMC free article] [PubMed] [Google Scholar]


