Abstract
Osteochondral injuries are common in humans and are relatively difficult to manage with current treatment options. The combination of novel biomaterials and expanded progenitor or stem cells provides a source of therapeutic and immunologically compatible medicines that can be used in regenerative medicine. However, such new medicinal products need to be tested in translational animal models using the intended route of administration in humans and the intended delivery device. In this study, we evaluated the feasibility of an arthroscopic approach for the implantation of biocompatible copolymeric poly-d,l-lactide-co-glycolide (PLGA) scaffolds in an ovine preclinical model of knee osteochondral defects. Moreover this procedure was further tested using ex vivo expanded autologous chondrocytes derived from cartilaginous tissue, which were loaded in PLGA scaffolds and their potential to generate hyaline cartilage was evaluated. All scaffolds were successfully implanted arthroscopically and the clinical evolution of the animals was followed by non invasive MRI techniques, similar to the standard in human clinical practice. No clinical complications occurred after the transplantation procedures in any of the animals. Interestingly, the macroscopic evaluation demonstrated significant improvement after treatment with scaffolds loaded with cells compared to untreated controls.
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-013-9581-3) contains supplementary material, which is available to authorized users.
Keywords: Preclinical animal model, Expanded chondrocytes, Regenerative medicine, Arthroscopy, Osteochondral defect
Introduction
Articular cartilage focal defects represent a common condition affecting the knee and play a significant role in the subsequent development of degenerative joint disease. The response to injury depends on the severity and depth of the lesion, but in general the healing potential is very limited. In this scenario, chondral lesions involving the subchondral bone usually result in fibrocartilage scar tissue refilling, which has poorer biomechanical and biochemical features compared to hyaline cartilage (Alford and Cole 2005).
Since the simultaneous regeneration of both cartilage and subchondral bone can be approached by the use of porous biopolymeric scaffolds that can contribute to (1) preserve the structure of the lesion area and (2) absorb bone marrow from the focal osteochondral injury, permitting bone remodelling and differentiation of neighbouring stem cells, we evaluated its use in a preclinical model of osteochondral injury. In particular, a large animal model was chosen in order to evaluate the viability of an arthroscopic approach for the implantation of a copolymeric poly-d,l-lactide-co-glycolide (PLGA) scaffold using standard instrumentation and techniques in human clinical practice. This is an important point, since there is a great deal of interest in the possibility of using arthroscopy in preclinical animal models for the development of new techniques and investigate the performance of novel implants in situ using minimally invasive approaches (Allen et al. 1998).
An osteochondral defect was created in the medial femoral condyle of the ovine stifle joint, since sheep is a representative animal model for studying a range of orthopaedic conditions and treatments because of its similar size and anatomy to the human knee. Although no animal model is completely ideal, sheep is a reasonable experimental animal for surrogate orthopaedic research directed at efficacy, safety, and mechanism of action (Simon and Aberman 2010) and, in fact, it has been previously used in several other studies for the treatment of chondral and osteochondral lesions (as reviewed in Ahern et al. 2009).
Materials and methods
Animals
The surgical procedures were developed in five cadavers that were euthanized for reasons unrelated to the current study. For the in vivo study, six healthy 2-year old ewes of the Ripollesa-Lacona breed were obtained from Servei de Granges i Camps Experimentals (SGCE, Bellaterra, Spain). During the pre- and post-operative procedures, animals were housed together, fed a standard diet and allowed access to water ad libitum. All animal care and experimental procedures were approved by the Universitat Autònoma de Barcelona’s Ethical Committee on Human and Animal Experimentation (Ref. No. CEAAH 501), and registered by the Departament de Medi Ambient de la Generalitat de Catalunya (Reg. No. 3666).
Study design
Two surgical procedures were performed in each animal. The first procedure involved an arthrotomy to harvest a cartilage biopsy for subsequent chondrocyte isolation and expansion (Suppl. Fig. 1A and 1B). The second procedure was performed after 4 weeks, and involved creation of a standardized osteochondral defect bilaterally in the medial femorotibial condyles via an arthroscopic approach (Suppl. Fig. 1D and 1F). Each of the osteochondral lesions produced in the 12 knees were included in one of any of the three treatments detailed next and described in Table 1: (a) 4 knees received scaffold seeded with expanded cells; (b) 4 knees received scaffolds without cells and (c) 4 knees were not treated. After surgery, sheep were allowed to move freely. Animals were divided in two identical groups, according to the two time points (12 and 20 weeks post-treatment, respectively).
Table 1.
Study design
| Animal ID | Time point (weeks) | Left knee | Right knee |
|---|---|---|---|
| F6 | 12 | S | CS |
| F12 | 12 | L | CS |
| F14 | 12 | S | L |
| F16 | 20 | CS | S |
| F17 | 20 | L | CS |
| F44 | 20 | L | S |
Experimental plan used to evaluate the effect of treatment with cell-seeded and cell-free PLGA scaffold to osteochondral defects on the medial femorotibial condyles
CS cell-seeded scaffold, S scaffold, L untreated lesion
Anaesthesia and post-operative care
All procedures were performed using aseptic techniques and under general anaesthesia. After premedication with an intramuscular (IM) injection of 0.01 mg/kg of buprenorphine (Buprex, Schering-Plough, S.A., Alcobendas, Spain) and 0.2 mg/kg of midazolam (Dormicum, Roche, Madrid, Spain), intravenous (IV) access was established at cephalic vein. Sheep were pre-oxygenated and induced with 4 mg/kg IV propofol (Propofol-Lipuro 1 %, BBraun Melsungen AG, Melsungen, Germany). The animals were under general anaesthesia intubated and maintained on isoflurane 2 % (Isoflo, Abbott laboratories Ltd, Abbott Park, IL, USA) with 100 % oxygen. Esophageal intubation was made to prevent ruminal bloat. A continuous infusion of Ringer lactate (Ringer lactate, BBraun Melsungen AG) was administered at 10 mL/kg/h during surgery. Intra-operative monitoring consisted of electrocardiography, pulse oximetry, non invasive blood pressure and capnography. All animals received one dose of subcutaneous (SC) meloxicam (Metacam, Boehringer-Ingelheim, Sant Cugat, Spain) 0.2 mg/kg daily for 10 days and a single dose of transdermal fentanyl (Durogesic, Janssen-Cilag, Madrid, Spain) 100 μg for post-operative pain relief. For peri-operative infection prophylaxis the animals received 22 mg/kg of cefazolin IM (Kurgan, Normon Laboratories, Madrid, Spain) every 12 h during 10 days.
Isolation, culture and scaffold-seeding of articular chondrocytes
The right shoulder joint was aseptically prepared and exposed with a craniolateral approach to the shoulder (Suppl. Fig. 1A). An approximately 3 cm-long incision was made in the skin and subcutaneous tissue, extending from the acromion process to the proximal humerus. The acromial portion of the deltoideus muscle was retracted caudally, and a lateral incision of the articular capsule was made between the glenoid rim and the humeral head. The humerus was rotated internally and an articular cartilage layer of 1 mm thick and 6 mm long was harvested from the head of humerus, using a curette. Cartilage was pierced and used as a source of chondrocytes (Suppl. Fig. 1). To do this, cartilage was washed and soaked with phosphate-buffered saline solution (PBS, HyClone, Logan, UT, USA) and immediately processed in the laboratory. The fragments of cartilage were minced, washed three times in PBS, and digested with 0.2 % (w/v) Collagenase B (Roche) for 24 h. Cells and tissue were resuspended in 10 mL of DMEM (Dulbecco’s modified Eagle’s medium, GIBCO-BRL/Life Technology, Carlsbad, CA, USA) supplemented with 10 % (v/v) foetal calf serum (FCS, Biological Industries, Kibbutz Beit Haemek, Israel) and centrifuged at 400 g for 5 min. Cells were washed in PBS and cultured in a T-25 flask in DMEM medium containing 10 % (v/v) FCS, in a humidified 5 % CO2 incubator. After cell attachment and mesenchymal-morphology conversion, cells were scaled-up by seeding T-150 flasks at 2,500 cell/cm2. Cell number and viability were determined by the haemocytometer-based trypan blue dye exclusion assay.
The cells were cultured in low glucose DMEM medium supplemented with Streptomycin (0.167 g/L, Sigma-Aldrich, St. Louis, MO, USA), Kanamycin (0.075 g/L, Sigma-Aldrich) and 10 % foetal calf serum (FCS, Biological Industries). Cells were passaged until passage 4 under a humidified atmosphere of 5 % carbon dioxide at 37 °C, using 0.25 % trypsin (GIBCO-BRL) and counted with a haemocytometer. The cultured chondrocytes obtained from each sheep were reseeded onto three-dimensional PLGA scaffolds in a minibioreactor (Hexascreen Culture Technologies S.L., Cerdanyola del Vallès, Spain) (Suppl. Fig. 1). Briefly, 3 × 106 cells were inoculated in each minibioreactor, where a PLGA scaffold was fixed using a 25G needle, and stirred at 200 rpm for 24 h incubation time in a total final volume of 12 mL. The amount of inoculum was determined in pilot experiments indicating the maximum load of cells absorbed by the scaffold (data not shown).
Scanning electron microscopy
Samples were fixed in 2.5 % glutaraldehyde and 2 % paraformaldehyde in PBS (pH 7) and dehydrated in ethanol series (15 min in each 30–50–70–90–100–100 %) to absolute ethanol and immediately transferred to acetone before being critical-point dried and gold-coated using a Sputter Coater (K550, Coating Attachment, Emitech, Ashford, U.K.). Samples were examined with an HITACHI S-570 electron microscope (Tokyo, Japan) at a voltage of 15 kV.
Scaffolds
PLGA scaffolds were manufactured in-house following a solution-casting/salt-leaching technique using PLGA particles with an inherent viscosity of 0.55–0.75 dL/g (Lactel) as described elsewhere (Mikos et al. 1994). Briefly, polymeric PLGA particles were first dissolved in chloroform. Then, sieved salt particles ranging 300–500 μm were dispersed in the polymer solution at a 9:1 ratio (NaCl:PLGA). The mixture was casted into a mould with a cylindrical shape, with a diameter of 4 mm and with 7 mm high. After the evaporation of the solvent, the salt particles were extracted by washing the polymer with distilled water for 48 h. Then the scaffolds were dried first at air temperature for 24 h and then vacuum dried for 24 h. Finally, the scaffolds were gamma sterilized (20 kGy) and stored at −20 °C.
Osteochondral defect creation and arthroscopic implantation
The creation of osteochondral defects and the introduction of the test items were performed arthroscopically (Suppl. Fig. 1). Each joint was approached via a stab incision lateral to the distal aspect of the patellar ligament, allowing the insertion of the arthroscope with camera for the visualization of the medial condyle. A mechanical shaver was used to remove the fat pad allowing a clearer view. For this purpose, a second stab incision was made medial to the distal aspect of the patellar ligament to allow the shaver insertion. Once cleaned, the shaver was removed. A mosaicplasty instrument set (Smith and Nephew Inc, Memphis, TN, USA), consisting of a donor and a receptor cannulae, was used to create the lesion and to place the scaffold (Suppl. Fig. 1D). For this purpose, the cylindrical hollow punch with an inner diameter of 2.7 mm (donor cannula) was placed through the second stab incision, previously used. This instrument was used to perform a cylindrical osteochondral lesion of diameter 3.5 and 5 mm depth in the caudal aspect of the medial femoral condyle of each knee. The scaffold of cylindrical shape and 4 mm diameter and 7 mm high was placed through the receptor canula, filling the lesion by the application of moderate force to the syringe piston (Suppl. Fig. 1F). The joints were irrigated by saline solution (NaCl-0.9, BBraun Medical SA) at room temperature during surgery.
Magnetic resonance imaging
MRI examination was performed on both knees of each animal under general anaesthesia, using a 0.2 T unit with open permanent magnet (Vet-MR, Esaote S.p.a., Genova, Italy). First group of animals was examined at 11 weeks post-treatment and the second group, at 19 weeks post-treatment.
Animals were positioned in sternal recumbency with the leg extended and symmetrically placed inside a dual phased array coil.
Sequences included axial, coronal and sagittal: sagittal high resolution spin echo T1 (TR/TE 590/26, DFOV 20 cm, slice thickness 4 mm) and high resolution spin echo T1 coronal (TR/TE 690/26, DFOV 22 cm, slice thickness 3 mm); sagittal sequences in Gradient echo (TR/TE 600/22, FA = 40º, DFOV 22 cm, slice thickness 4 mm); sagittal STIR imaging (TR/TE/TI 1560/24/75, DFOV 23 cm, slice thickness 4 mm) and coronal STIR imaging (Short T1 Inversion Recovery) for fat suppression (TR/TE/TI 1840/24/75, DFOV 23 cm, slice thickness 3 mm); axial PD/T2 Turbo Multiecho imaging (TR/TE 2800/28 and 2800/90, DFOV 22, slice thickness 4 mm).
Macroscopic assessment
The animals from the two experimental groups were sedated with an IM injection of 0.02 mg/kg of midazolam and euthanised by IV injection of 12 mg/kg pentobarbital (Dolethal 20 mg/100 mL, Vetoquinol S.A., Paris, France).
Both stifle joints were harvested, grossly evaluated blindly by CF, JB and FG, and photographed. The gross appearance of repaired tissues was assessed in accordance with the International Cartilage Repair Society (ICRS) classification (van den Borne et al. 2007). Biologically acceptable regeneration was defined as having smooth, firm repair tissue that filled the defect and appeared attached to the adjacent cartilage. Samples were excised in blocks including all the implanted tissue and the surrounding native tissue, and were fixed in a 4 % PFA (paraformaldehyde, Sigma-Aldrich) solution, for histological analysis.
Histological evaluation and immunohistochemical procedures
Condyles were fixed in 4 % PFA solution at room temperature for 2 weeks, decalcified with 5 % formic acid for 4–6 weeks and embedded in paraffin for subsequent histological and immunohistochemical procedures. Microtome sections 4 μm thick were cut in sagittal plane sections and stained with haematoxylin/eosin (H&E, Sigma-Aldrich) and Safranin O (Sigma-Aldrich). The success of the treatment was evaluated blindly by PJF following the Histological scoring Grading Scale on at least 4 sequential histological sections (Mainil-Varlet et al. 2001).
The presence of Collagen type II and Sox9 was detected by immunohistochemical techniques using polyclonal antibodies (MAB 8887, Chemicon (EMD Millipore Corporation, Billerica, MA, USA); and AB5535, Chemicon, respectively). Briefly, paraffin sections were deparaffinized and hydrated. For collagen type II, sections were also treated with 0.1 % Pepsin (Sigma-Aldrich) in HCl 0.01N for 20 min. For both collagen type II and sox9, endogenous peroxidase activity was blocked with hydrogen peroxide for 30 min and washed in 3 % bovine serum albumin (BSA, Sigma-Aldrich) in Phosphate Buffered Saline (PBS, Sigma-Aldrich). The sections were then incubated with either the antibody against collagen type II (dilution 1:200) or sox9 (dilution 1:200) overnight at 4 °C. Antibody binding was visualized by using Universal LSAB™ 2 Kits (Dako, Glostrup, Denmark) in combination with diaminobenzidine (DAB) according to the manufacturer’s instructions. The sections were counterstained with haematoxylin.
Statistical analysis
To verify the comparability of the treatment groups, comparisons with regard to macroscopic evaluation and histological grade were performed using a non-parametric Kruskal–Wallis statistical analysis (n = 4) with the GraphPad Prism program (GraphPad software).
Results
Production of scaffolds and cell/scaffold constructs
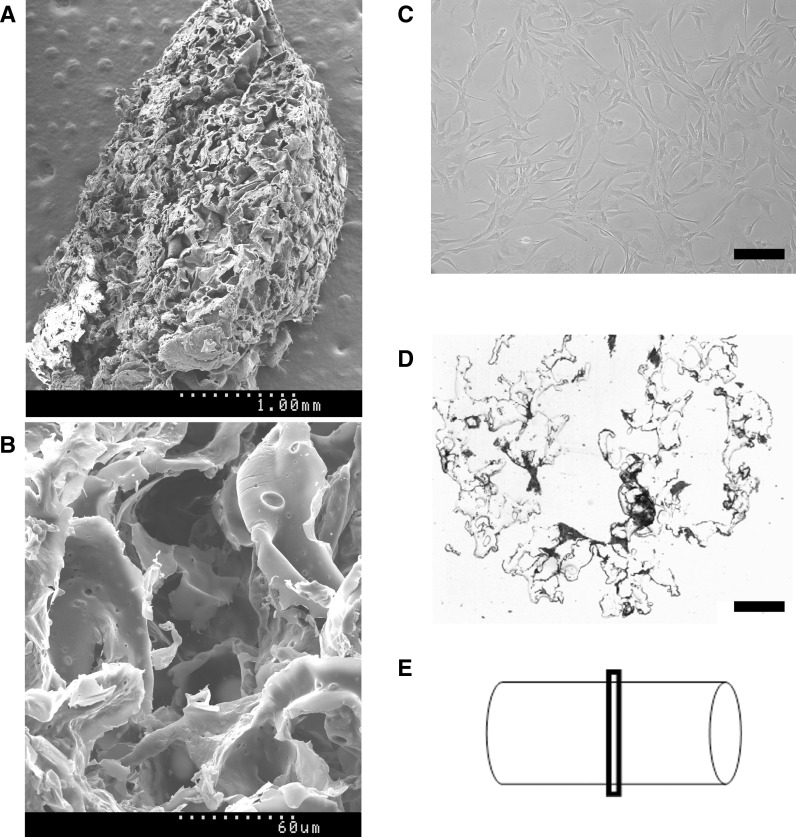
Scanning electron microscopy was used to assess the microstructure of the porous PLGA scaffolds produced. They displayed high interconnectivity of pores, and a 3D structure that facilitated migration of cells within the scaffold (Fig. 1a, b).
Fig. 1.
SEM images from macroporous PLGA scaffolds. a, b Cell-free scaffold at low and high magnification, respectively. c Ovine chondrocytes in culture are stretched cells with fibroblastic appearance. d Cross-section from an H&E stained cell-seeded scaffold, as shown in e, where the schematic shows the structure of the scaffold with a cylindrical shape of 4 mm diameter and 7 mm long. Scale bars c = 200 μm; d = 600 μm
Autologous chondrocytes were expanded in vitro yielding a therapeutic dose of 3 × 106 cells/scaffold (Fig. 1c). Each PLGA scaffold and its corresponding dose of chondrocytes were incubated together in a single-use bioreactor resulting in colonization rates of more than 70 %: that is a number of cells seeded ranging between 2.2 × 106 and 2.4 × 106 cells/scaffold, as determined by indirect counting of non-seeded cells present on the supernatant. Histological analysis of seeded scaffolds showed cells consistently distributed within the PLGA structure and tightly attached to the surface (Fig. 1d).
Experimental lesion, test item implantation and clinical outcome
Bilateral arthroscopy was well tolerated and all animals were able to stand on all four limbs and walk immediately after surgery. No clinical complications occurred after the transplantation procedures in any of the animals.
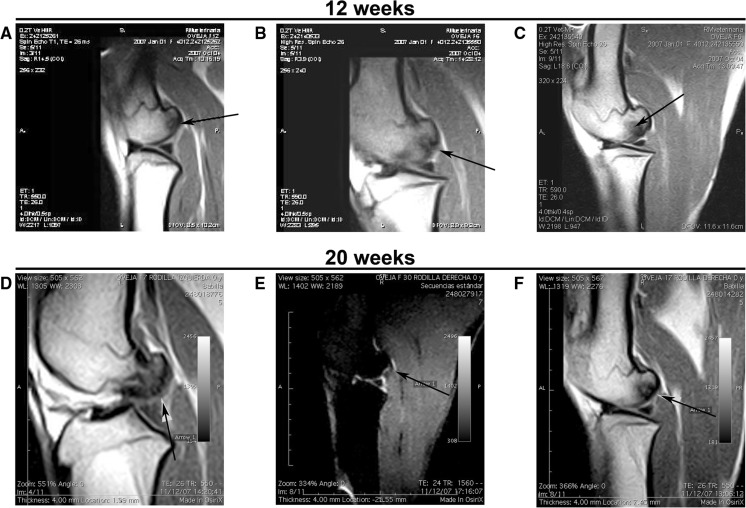
MRI evaluation at 11 and 19 weeks (prior to euthanasia at 12 and 20-week post-surgery, respectively) revealed osteochondral sclerotic lesions in all untreated knees (Fig. 2a, d). Most of the defects treated with scaffold constructs, either with or without cells, presented continuity of the surface, which was not observed in the untreated group (Fig. 2).
Fig. 2.
MRI images. The top images were taken at 12 weeks after arthroscopy: a untreated knee (sample F12left). b Defect treated with cell-free PLGA scaffold (sample F6left), where an incomplete osteointegration of the implant is shown by an arrow; and c osteochondral defect treated with cell-seeded PLGA scaffold (sample F6right), where the arrow points to an oedema-like signal in the subchondral bone. Bottom images were taken from samples recovered 20 weeks post arthroscopy: d untreated knee (sample F17left), where the arrow points at the irregular articular surface. e Lesion treated with cell-free PLGA scaffold (sample F30right), where the arrow points to the erosion on the articular surface; and f lesion treated with cell-seeded PLGA scaffold (sample F17right), where the arrow indicates the continuity of the surface
Macroscopic assessment and histological results
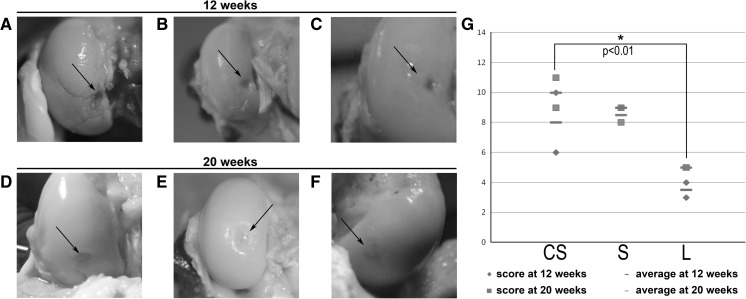
Animals were euthanized at 12 and 20 weeks post-treatment, and the repair process was evaluated macroscopically. No evidence of infection or fibrinous overgrowth on the joint surface was observed. Taken together, both scores from groups euthanized at 12 and 20 weeks, knees treated with cell-seeded scaffolds presented the best macroscopic results and showed differences that were statistically significant (p < 0.01) compared to untreated controls (CS = 9.0 ± 2.1; S = 8.8 ± 0.5; L = 4.3 ± 1.0; Fig. 3g).
Fig. 3.
Macroscopic aspect of knees. The top images were taken at 12 weeks post arthroscopy: a knee displaying a condyle (sample F12left) suffering arthritis as a consequence of the untreated osteochondral lesion; b lesion treated with cell-free PLGA scaffold and showing a good integration of the implant (sample F6left) and merely displaying a slight erosion on the surface; and c defect treated with cell-seeded PLGA scaffold (sample F6right), where the arrow marks the integration site of the repair tissue next to the border zone of the native tissue, which appears almost identical. Bottom images show macroscopic findings at 20 weeks post arthroscopy: d untreated lesion in sample F17left, where the arrow marks the defect site; e lesion treated with cell-free PLGA scaffold in sample F30right, where a repair tissue (arrow) appears below the level of the native articular cartilage; and f cell-seeded PLGA scaffold treatment in sample F17r, offering repair tissue well integrated (arrows), and levelled with the surrounding native articular cartilage. g Graphical representation of the scores obtained at 12 and 20 weeks grouped together according to each treatment, where the average from each group is also graphically represented to highlight the similarity in their values. A significant statistical difference (p < 0.01) was observed between the group treated with cell-seeded scaffold compared to the untreated lesion. CS cell-seeded scaffold, S scaffold, L untreated lesion
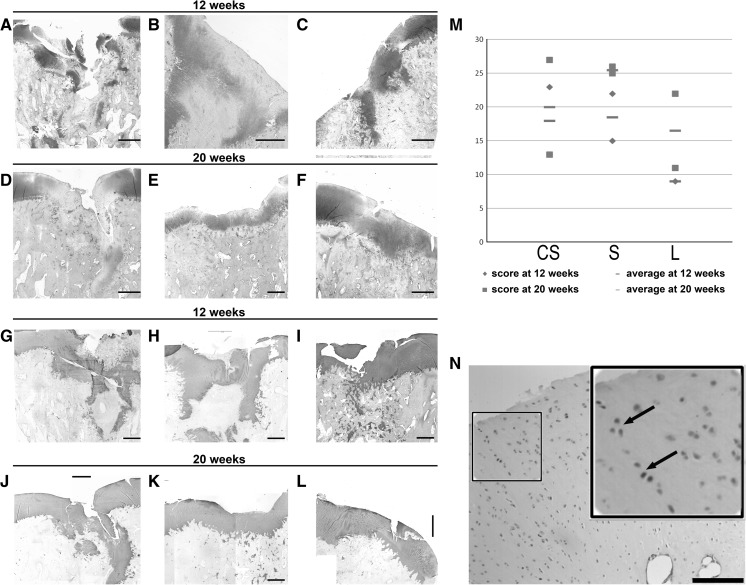
At the histological level, a repetitive pattern was observed on the surface of the perilesional zone in all samples, consisting in a marked decrease in cellular density, low intensity of Safranin O staining and presence of clusters of chondrocytes, preferentially in the most superficial layers and the more numerous, the closer to the area of the lesion (Fig. 4).
Fig. 4.
Histochemical analysis of paraffin sections of cartilage specimens. In the left top panels, images from samples taken at 12 and 20 weeks post-operatively stained with Safranin O. In the left bottom panels, Collagen II immunostainings from samples taken at 12 and 20 weeks post-operatively. a, g Untreated knee (sample F12left); b, h defect treated with cell-free PLGA scaffold (F14left); c, i defect treated with cell-seeded PLGA scaffold (sample F6right); d, j untreated knee (sample F17left); e, k defect treated with cell-free PLGA scaffold (F16right); f, l defect treated with cell-seeded PLGA scaffold (sample F17right). m Graphical representation of the scores obtained at 12 and 20 weeks grouped together according to each treatment, where the average from each group is also graphically represented to highlight the similarity in their values. n Section from a knee treated with PLGA scaffolds loaded with chondrocytes (sample F16left) and stained for Sox9 (inset: zoomed view with arrows pointing to Sox9 positive cells). CS cell-seeded scaffold, S scaffold, L untreated lesion. Scale bar 600 μm
Although macroscopically fibrous tissue was only observed in specimens from the 20-week time point, all 12 and 20-week untreated knees displayed at the histological level some amount of fibrous repair tissue of low quality lacking continuity of the cartilage surface surrounding the damaged area.
Collagen II positive immunostaining displayed a uniform sheet pattern distribution on the superficial cartilage in those lesions that were treated with cell-free scaffolds (at 20 weeks post-treatment; Fig. 4k) and cell-seeded scaffolds (both at 12 and 20-week post-treatment; Fig. 4i, l). Expression of Sox9 was observed in cells present within the hyaline cartilage (Fig. 4n). In all samples, Sox9 signal was found in most superficial cells of native hyaline cartilage and in clusters of chondrocytes located superficially in the perilesional area.
Taken together the scores from the histology and immunohistochemistry for each treatment, no statistically significant differences (p > 0.01) were found among any of the experimental groups (CS = 19.0 ± 7.1; S = 22.0 ± 5.0; L = 10.5 ± 9.0; Fig. 4m).
Discussion
In the orthopaedic field, special attention is given to cartilage repair using tissue-engineering techniques due to the complexity of pathologies involving two types of distinct tissues: articular cartilage and subchondral bone. Accordingly, the use of either natural based polymers or synthetic materials in the manufacture of biocompatible scaffolds is currently a hot topic. Indeed, numerous studies have focused on the design of complex scaffolds, including the combination of two distinct layers or a gradient scaffold (reviewed by Rodrigues et al. 2011). In the present study, PLGA was chosen because it is one of the few synthetic materials approved by the FDA as scaffolding material for clinical applications and it has been previously used in articular cartilage treatment, emerging as a valuable chondrocyte and MSC delivery vehicle (Sittinger et al. 1996; Uematsu et al. 2005). Furthermore, the method followed for the manufacture of the scaffolds permitted to set a porosity level that allowed the ingrowth of host tissue as well as supporting the preloading with in vitro-expanded chondrocytes. Importantly, the scaffold structure allowed arthroscopic implantation making it an attractive material for clinical use and highlights the need for the use of large animal models in the development of new cartilage repair techniques; in particular, those species with size and physiology similar to humans. We used skeletally mature 2-year-old sheep (Kilborn et al. 2002), and the osteochondral defects were modelled in weight bearing areas. For this purpose, the experimental defects were located in the central/posterior region of the medial femoral condyle, with the aim of represent the clinical situation faced in humans. We chose to harvest cartilage cells from the shoulder, so both knees of each animal could be used in the study, in the same comparable conditions without additional lesions on the surrounding area that might influence the outcome of the treatments.
The use of chondrocytes allowed a significant improvement with respect to untreated controls at the macroscopic level, although this observation was not confirmed histologically, probably due to the short observation time. Therefore, it was not clear whether the addition of chondrocytes into the PLGA scaffolds could significantly accelerate the regeneration mechanisms.
To our knowledge, this is the first report of arthroscopic implantation of the PLGA scaffolds in the sheep knee. Published studies used arthrotomy procedures to implant the tissue engineered constructs (Córdoba et al. 2007; Erggelet et al. 2007; Niederauer et al. 2000), because the difficulty of holding in place the implant without invasive procedures. The biomechanical characteristics of some of the constructs, as the ones made of chondrocytes and fibrin are too soft to hold in the defect site independently of the use of a periosteum patch. The need of development of this minimally invasive technique in the sheep knee has been mentioned by some authors (Munirah et al. 2007; Sha’ban et al. 2008) and the regulatory authorities require the use of the intended route of administration in humans and the intended delivery device in preclinical studies. This surgical technique has the recognized advantage of decreased morbidity associated with arthrotomy, more ethical experimental procedure and a final study that matches the clinical situation faced in human patients. On the other side there are two limitations that need to be acknowledged in the present study. Although the clinically accepted range of MRI covers from 0.2T to 3T (Ghazinoor et al. 2007), the use of low-field MRI in this study made the detection of small cartilage abnormalities very challenging. The second point concerns to the sample size and the proximity of the two time endpoints for the euthanasia, which gave similar results, making possible to combine data for each treatment from both the 12- and 20-week groups for the statistical analysis. Future studies should evaluate the effects of a full regeneration process at later end-points (i.e., up to 12 months), other cell sources, and the appropriate therapeutic dose.
Electronic supplementary material
Supplementary material Suppl. Fig. 1. Methodology for chondrocyte isolation, scaffold loading and arthroscopic implantation. A) Arthrotomy of the shoulder for articular cartilage sample isolation which was immediately collected (B) in a Falcon tube; (C) expanded cells were inoculated in a bioreactor containing PLGA scaffold; (D) mosaicplasty set, with donor and receptor cannulae; (E) surgeon removing the PLGA scaffold from the bioreactor, prior to the implantation; (F) arthroscopy portals in the sheep knee, for PLGA scaffold implantation. (TIFF 45585 kb)
Acknowledgments
The authors would like to acknowledge critical review and helpful comments of the original manuscript by Dr. Joan Garcia; Anna Morist, Anna Garrit and Cristian de la Fuente for technical assistance; and José Luís Ruiz, Ramón Costa and the crew of the “Servei de Granges i Camps Experimentals” of the UAB (Bellaterra, Spain) for their careful assistance to animal management. The project MEDCEL (PSE-010000-2007-4) was supported by the Spanish Ministry of Education and Science (MEC).
Footnotes
C. Fonseca, M. Caminal and D. Peris contributed equally to this work.
Contributor Information
A. Pla, Phone: +34-93-5573500, Email: apla@bst.cat
J. Vives, Phone: +34-93-5573500, Email: jvives@bst.cat
References
- Ahern BJ, Parvizi J, Boston R, Schaer TP. Preclinical animal models in single site cartilage defect testing: a systematic review. Osteoarthr Cartil. 2009;17:705–713. doi: 10.1016/j.joca.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33:295. doi: 10.1177/0363546504273510. [DOI] [PubMed] [Google Scholar]
- Allen MJ, Houlton JEF, Adams SB, Rushton N. The surgical anatomy of the stifle joint in sheep. Vet Surg. 1998;27:596–605. doi: 10.1111/j.1532-950X.1998.tb00536.x. [DOI] [PubMed] [Google Scholar]
- Córdoba FEV, Martínez CV, López VM, Butrón HL, Marín BR, Villaseñor EE, Castrejón HV, Arrieta LS, Morales RE, de León CIP. Resultados en la reparación experimental de lesiones osteocondrales en un modelo porcino mediante ingeniería de tejidos. Acta Ortopédica Mexicana. 2007;21:217–223. [PubMed] [Google Scholar]
- Erggelet C, Neumann K, Endres M, Haberstroh K, Sittinger M, Kaps C. Regeneration of ovine articular cartilage defects by cell-free polymer-based implants. Biomaterials. 2007;28:5570–5580. doi: 10.1016/j.biomaterials.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Ghazinoor S, Crues JV, 3rd, Crowley C. Low-field musculoskeletal MRI. J Magn Reson Imaging. 2007;25:234–244. doi: 10.1002/jmri.20854. [DOI] [PubMed] [Google Scholar]
- Kilborn SH, Trudel G, Uhthoff H. Review of growth plate closure compared with age at sexual maturity and lifespan in laboratory animals. Contemp Top Lab Anim Sci. 2002;41:21–26. [PubMed] [Google Scholar]
- Mainil-Varlet P, Rieser F, Grogan S, Mueller W, Saager C, Jakob RP. Articular cartilage repair using a tissue-engineered cartilage-like implant: an animal study. Osteoarthr Cartil. 2001;9:S6–S15. doi: 10.1053/joca.2001.0438. [DOI] [PubMed] [Google Scholar]
- Mikos AG, Thorsen AJ, Czerwonka LA, Bao Y, Langer R, Winslow DN, Vacanti JP. Preparation and characterization of poly(l-lactic acid) foams. Polymer. 1994;35:1068–1077. doi: 10.1016/0032-3861(94)90953-9. [DOI] [Google Scholar]
- Munirah S, Samsudin OC, Chen HC, Salmah SH, Aminuddin BS, Ruszymah BH. Articular cartilage restoration in load-bearing osteochondral defects by implantation of autologous chondrocyte–fibrin constructs: an experimental study in sheep. J Bone Joint Surg Br. 2007;89:1099–1109. doi: 10.1302/0301-620X.89B8.18451. [DOI] [PubMed] [Google Scholar]
- Niederauer GG, Slivka MA, Leatherbury NC, Korvick DL, Harroff HH, Ehler WC, Dunn CJ, Kieswetter K. Evaluation of multiphase implants for repair of focal osteochondral defects in goats. Biomaterials. 2000;21:2561–2574. doi: 10.1016/S0142-9612(00)00124-1. [DOI] [PubMed] [Google Scholar]
- Rodrigues MT, Gomes ME, Viegas CA, Azevedo JT, Dias IR, Guzon FM, Reis RL. Tissue-engineered constructs based on SPCL scaffolds cultured with goat marrow cells: functionality in femoral defects. J Tissue Eng Regen Med. 2011;5:41–49. doi: 10.1002/term.287. [DOI] [PubMed] [Google Scholar]
- Sha’ban M, Kim SH, Idrus RB, Khang G. Fibrin and poly(lactic-co-glycolic acid) hybrid scaffold promotes early chondrogenesis of articular chondrocytes: an in vitro study. J Orthop Surg Res. 2008;3:17. doi: 10.1186/1749-799X-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon TM, Aberman HM. Cartilage regeneration and repair testing in a surrogate large animal model. Tissue Eng B Rev. 2010;16:65–79. doi: 10.1089/ten.teb.2009.0304. [DOI] [PubMed] [Google Scholar]
- Sittinger M, Reitzel D, Dauner M, Hierlemann H, Hammer C, Kastenbauer E, Planck H, Burmester GR, Bujia J. Resorbable polyesters in cartilage engineering: affinity and biocompatibility of polymer fiber structures to chondrocytes. J Biomed Mater Res. 1996;33:57–64. doi: 10.1002/(SICI)1097-4636(199622)33:2<57::AID-JBM1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Uematsu K, Hattori K, Ishimoto Y, Yamauchi J, Habata T, Takakura Y, Ohgushi H, Fukuchi T, Sato M. Cartilage regeneration using mesenchymal stem cells and a three-dimensional poly-lactic-glycolic acid (PLGA) scaffold. Biomaterials. 2005;26:4273–4279. doi: 10.1016/j.biomaterials.2004.10.037. [DOI] [PubMed] [Google Scholar]
- van den Borne MPJ, Raijmakers NJH, Vanlauwe J, Victor J, de Jong SN, Bellemans J, Saris DBF. International Cartilage Repair Society (ICRS) and Oswestry macroscopic cartilage evaluation scores validated for use in autologous chondrocyte implantation (ACI) and microfracture. Osteoarthr Cartil. 2007;15:1397–1402. doi: 10.1016/j.joca.2007.05.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material Suppl. Fig. 1. Methodology for chondrocyte isolation, scaffold loading and arthroscopic implantation. A) Arthrotomy of the shoulder for articular cartilage sample isolation which was immediately collected (B) in a Falcon tube; (C) expanded cells were inoculated in a bioreactor containing PLGA scaffold; (D) mosaicplasty set, with donor and receptor cannulae; (E) surgeon removing the PLGA scaffold from the bioreactor, prior to the implantation; (F) arthroscopy portals in the sheep knee, for PLGA scaffold implantation. (TIFF 45585 kb)