Abstract
Spondias pinnata, a commonly distributed tree in India, previously proven for various pharmacological properties and also reported for efficient anti-oxidant, free radical scavenging and iron chelating activity, continuing this, the present study is aimed to investigate the role of 70 % methanolic extract of S. pinnata bark (SPME) in promoting apoptosis in human lung adenocarcinoma cell line (A549) and human breast adenocarcinoma cell line (MCF-7). These two malignant cell lines and a normal cell line were treated with increasing concentrations of SPME and cell viability is calculated. SPME showed significant cytotoxicity to both A549 and MCF-7 cells with an IC50 value of 147.84 ± 3.74 and 149.34 ± 13.30 μg/ml, respectively, whereas, comparatively no cytotoxicity was found in normal human lung fibroblast cell line (WI-38): IC50 932.38 ± 84.44 μg/ml. Flow cytometric analysis and confocal microscopic studies confirmed that SPME is able to induce apoptosis in both malignant cell lines. Furthermore, immunoblot result proposed the pathway of apoptosis induction by increasing Bax/Bcl-2 ratio in both cell types, which results in the activation of the caspase-cascade and ultimately leads to the cleavage of Poly adeno ribose polymerase. For the first time this study proved the anticancer potential of SPME against human lung and breast cancer by inducing apoptosis through the modulation of Bcl-2 family proteins. This might take S. pinnata in light to investigate it for further development as therapeutic anticancer source.
Keywords: Spondias pinnata, Anticancer, Apoptosis, Caspase, Bax/Bcl-2
Introduction
Cancer is a major public health issue with millions of new patients diagnosed each year and many deaths resulting from this disease. Cancer is a net accumulation of abnormal cells, which can arise from an excess of proliferation or an inefficiency of undergoing apoptosis or the combination of both. Manifestations of apoptosis are easily noticeable by the appearance of cell shrinkage, membrane blebbing, chromatin condensation, DNA cleavage, and finally, fragmentation of the cell into membrane-bound apoptotic bodies.
Thus, in cancer therapy, the focus is on strategies that suppress tumor growth by activating the apoptotic program in the cell. Till date chemotherapy remains the principal mode of treatment for various types of cancers including breast and lung cancer. Tamoxifen, a-non steroidal anti-estrogen drug, is used in the treatment of breast cancer patients and as chemoprevention in high risk women (Fisher et al. 2005). The anthracycline doxorubicin is regularly used as a chemotherapeutic agent in the treatment of lung and breast cancer (Lin et al. 2012; Moreno-Aspitia and Perez 2009). Conversely, the development of resistance to chemotherapeutic drugs obstructs effective killing of the cancer cells, resulting in tumor recurrence. In addition, patients usually suffer from serious side-effects such as cardiac and other toxicities (Christiansen and Autschbach 2006; Wonders and Reigle 2009). So, the development of better and safer drug from natural resources to cure cancer still remains a big challenge for the scientific community.
Spondias pinnata (Linn. f.) Kurz (Family—Anacardiaceae) is a deciduous tree widely distributed in India, Sri Lanka and South-East Asian countries. In India, it is seen in the deciduous to semi-evergreen forests. The gum exudate of the species has been reported to contain acidic polysaccharides (Ghoshal and Thakur 1981). In ethnomedicine, S. pinnata is used for its antibacterial activity (Bibitha et al. 2002), treatment of dysentery (Mahanta et al. 2006) and inhibits the citrus canker of lime (Leksomboon et al. 2001). It is also reported that the bark of S. pinnata acts as a very good antioxidant and free radical scavenger (Hazra et al. 2008) as well as a potent iron chelator properties (Hazra et al. 2013). However, there has been no report on the anticancer properties of this species.
It will be much of interest if the anticancer and apoptosis inducing property of S. pinnata bark can be established. Therefore, the present study is aimed to demonstrate the in vitro anticancer activity of 70 % methanolic extract of Spondias pinnata bark (SPME) on human lung carcinoma A549 and on human breast carcinoma MCF-7 and to investigate the apoptosis inducing property by studying morphological changes, cell cycle analysis and by checking the expression of anti and proapoptotic proteins as well as a caspase cascade pathway.
Materials and methods
Chemicals
Ham’s F-12, Dulbecco’s Modified Eagle’s Medium (DMEM), antibiotics and Amphotercin-B were purchased from HiMedia Laboratories Pvt. Ltd. (Mumbai, India). Fetal bovine serum was purchased from HyClone Laboratories, Inc. (Logan, UT, USA). Cell Proliferation Reagent WST-1, Annexin-V-FLUOS staining kit and Polyvinyl difluoride membrane were purchased from Roche Diagnostics (Mannheim, Germany). RNase A, 4′,6′-diamidino-2 phenylindole and Triton X-100 were purchased from MP Biomedicals (Illkirch Graffenstaden, France). Non-Fat dry milk was purchased from Mother Dairy, G. C. M. M. F. Ltd. AMUL (Anand, Gujarat, India). BCIP/NBT substrate was purchased from GeNei™, MERCK (Mumbai, India). Anti-PARP N-terminus, anti-Bcl-2 (NT), anti-Caspase-3 (p17), anti-Caspase-9 and anti-Caspase-8 (IN) antibodies were purchased from AnaSpec, Inc. (Fremont, CA, USA). Anti-Bax and anti-beta-actin antibodies were purchased from OriGene Technologies, Inc (Rockville, MD, USA). Alkaline phosphatase conjugated anti-Rabbit secondary antibody was purchased from RockLand Immunochemicals Inc. (Gilbertsville, PA, USA).
Plant material
The stem bark of the S. pinnata was collected from the Bankura district of West Bengal, India. The plant was identified by the Central Research Institute (Ayurveda), Kolkata, India, where a specimen of the plant was deposited (Specimen No. CRHS 111/08).
Extraction
The powder (100 g) of air-dried stem bark of S. pinnata was stirred using a magnetic stirrer with a 70 % methanol in water (1,000 ml) for 15 h; the mixture was then centrifuged at 2,850×g and the supernatant was decanted. The process was repeated by adding the same solvent with the precipitated pellet. The supernatants from two phases were mixed, concentrated in a rotary evaporator and lyophilized. The obtained dried extract was stored at −20 °C until use.
Sample preparation
The working stock solution (20 mg/ml) of S. pinnata bark extract was prepared using distilled water and sterilised using 0.22 μm syringe filter. The obtained sample solution was stored at 4 °C until use.
Cell line and culture
Human lung adenocarcinoma (A549), human breast adenocarcinoma (MCF-7) and human lung fibroblast (WI-38) cell lines were purchased from the National Centre for Cell Science (NCCS, Pune, India) and maintained in the laboratory. A549 cells were grown in Ham’s F-12 medium whereas MCF-7 and WI-38 cells were grown in DMEM. Both media were supplemented with 10 % (v/v) fetal bovine serum (FBS), 100 U/ml Penicillin G, 50 μg/ml Gentamycin sulphate, 100 μg/ml Streptomycin and 2.5 μg/ml Amphotericin B. All cell lines were maintained at 37 °C in a humidified atmosphere containing 5 % CO2 in CO2 incubator.
WST-1 cell proliferation assay
Cell proliferation and cell viability were quantified using the WST-1 Cell Proliferation Reagent, Roche Diagnostics, according to the manufacturer’s instructions. The principle of this assay is based on cleavage of a tetrazolium salt to a formazan by cellular enzymes, especially mitochondrial dehydrogenases (Mosmann 1983). The number of metabolically active cells correlates directly to the amount of formazan.
A549 cells were seeded in a 96-well culture plate at a density of 5 × 104 cells/well whereas MCF-7 cells and WI-38 cells were seeded at 1 × 104 cells/well and allowed to settle for 2 h. The cells were then treated with SPME ranging from 0 to 200 μg/ml for 48 h. After treatment, 10 μl of WST-1 cell proliferation reagent was added to each well followed by 3–4 h of incubation at 37 °C. Cell proliferation and viability were quantified by measuring the absorbance of the formazan at 460 nm using a microplate ELISA reader MULTISKAN EX (Thermo Electron Corporation, Waltham, MA, USA).
Cell cycle analysis
Cell cycle analysis was performed by flow cytometry using the method previously described with slight modifications (Sarkar and Mandal 2011). Before treatment with SPME (0–200 μg/ml), A549 and MCF-7 cells were seeded in 6 well plates at a density of 1.5 × 106 cells/well and allowed to adhere to surface for 12 h. After 16 h of treatment, cells were harvested and fixed with suitable amount of chilled methanol and diluted with PBS. Cells were then treated with RNase A at 37 °C for 1 h to digest cellular RNA. The nuclear DNA of cells was then stained with propidium iodide and cell phase distribution was determined on FACS Calibur (Becton–Dickinson, Franklin Lakes, NJ, USA) equipped with a 488 nm Argon laser light and a 623 nm band pass filter using CellQuest software. A total of 10,000 events were acquired and data analysis was done using the ModFit software. A histogram of DNA content (x-axis, red fluorescence) versus count (y-axis) was plotted.
Annexin V staining
This assay was performed using the Annexin-V-FLUOS Staining kit (Roche Diagnostics). The cells (1 × 106) were treated with SPME (0–200 μg/ml) for 16 h labelled with PI and FITC according to the protocol of the kit manufacturer. The distribution of apoptotic cells was identified by flow cytometry on a FACS Calibur (Becton–Dickinson) equipped with a 488 nm Argon laser light and a 623 nm band pass filter using the CellQuest software. A total of 10,000 events were counted. Cells that were Annexin V (−) and PI (−) were considered as viable cells. Cells that were Annexin V (+) and PI (−) were considered as early stage apoptotic cells. Cells that were Annexin V (+) and PI (+) were considered as late apoptotic or necrotic cells.
DAPI (4′, 6′-diamidino-2 phenylindole) staining
DAPI staining was done using the method described earlier with slight modifications (Machana et al. 2011). In brief, A549 and MCF-7 cells were seeded in 6-well culture plates containing 22 mm glass coverslip at a density of 5 × 105 cells/well. The cells were then allowed to adhere to surface for 16 h followed by treatment with SPME (80 and 100 μg/ml). After 48 h of treatment, cells were fixed with 4 % paraformaldehyde followed by permeabilization with 0.1 % Triton X-100. Cells were stained with 50 μg/ml DAPI for 40 min at room temperature. The cells undergoing apoptosis, represented by the morphological changes of apoptotic nuclei, were observed and imaged from ten eye views at 63× magnifications under a laser scanning confocal microscope LSM510META (Zeiss, Oberkochen, Germany).
Western blot analysis
1 × 106 cells were treated with SPME (80 μg/ml for A549 and 100 μg/ml for MCF-7) for various time intervals (0.5–24 h). After treatment, cells were lysed with triple detergent cell lysis buffer (50 mM Tris–Cl, 150 mM NaCl, 0.02 % Sodium azide, 0.1 % Sodium dodecyl sulphate, 1 % Triton X-100, 0.5 % sodium deoxycholate, 1 μg/ml aprotinin, 100 μg/ml phenyl methyl-sulfonyl fluoride, pH 8) and the lysates were then centrifuged at 13,800×g for 20 min at 4 °C. The supernatants were stored at −80 °C until use. Protein concentration was measured by the Folin-Lowry method. Proteins (50 μg) in the cell lysates were resolved on 12 % SDS-PAGE for caspase-9, Caspase-3 and Bax whereas 40 μg of protein were used to resolve Caspase-8, Bcl-2, PARP and beta-actin on 10 % SDS-PAGE. The proteins were transferred to the PVDF membrane using transfer buffer (39 mM Glycine, 48 mM Tris base, 20 % Methanol, 0.037 % Sodium dodecyl sulphate, pH 8.3). The membranes were then blocked with 5 % Non-fat dry milk in TBS followed by incubation with corresponding antibodies separately overnight at 4 °C. After washing with TBS-T (0.01 % of Tween-20 in TBS) membranes were incubated with alkaline phosphatase conjugated anti-Rabbit IgG antibody at room temperature in the dark for 4 h, followed by washing. The blots were then developed with BCIP/NBT substrate and the images were taken by the imaging system EC3 Chemi HR (UVP, Upland, CA, USA). The blots were then analysed for band densities using ImageJ 1.45 s software.
Statistical analysis
Cytotoxicity data were reported as the mean ± SD of 6 measurements and cell cycle analysis data were reported as the mean ± SD of 3 measurements. The statistical analysis was performed by KyPlot version 2.0 beta 15 (32 bit). The IC50 values were calculated by the formula, Y = 100*A1/(X + A1) where A1 = IC50, Y = response (Y = 100 % when X = 0), X = inhibitory concentration. The IC50 values were compared by paired t test. p < 0.05 was considered significant.
Results
Cell proliferation assay
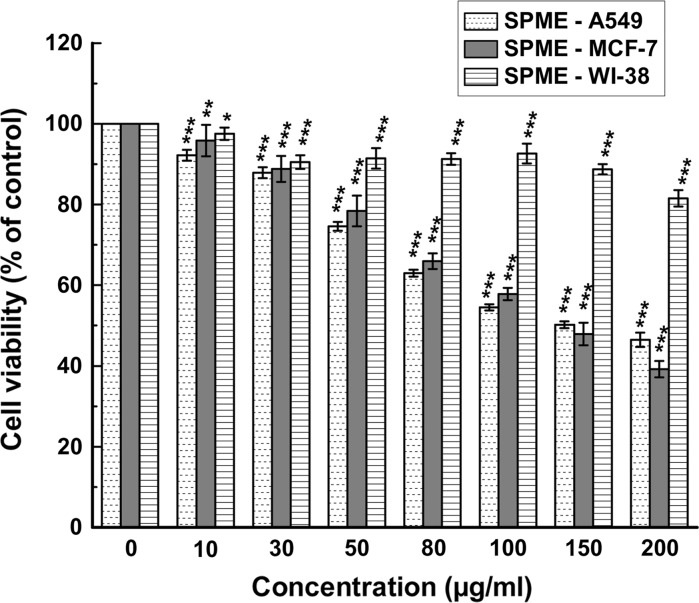
The effect of SPME on cell viability was evaluated individually by WST-1 assay and the IC50 values were calculated from dose-dependent response studies assessed 48 h post-treatment. SPME inhibited the growth of both A549 and MCF-7 cells in a dose-dependent manner with an IC50 value of 147.84 ± 3.74 and 149.34 ± 13.30 μg/ml, respectively. The cytotoxicity of SPME on the normal fibroblast cell line was also evaluated, the results showed that treatment of WI-38 with the extract did not inhibit the cell proliferation significantly and the IC50 was calculated as 932.38 ± 84.44 μg/ml (Fig. 1).
Fig. 1.
Effect of SPME on cell proliferation and viability of A549, MCF-7 and WI-38 cells. Cells were treated with increasing concentrations of SPME for 48 h; cell proliferation and viability were determined with WST-1 cell proliferation reagent. Results were expressed as cell viability (% of control). All data are expressed as mean ± SD (n = 6). *p < 0.05, **p < 0.01 and ***p < 0.001 versus 0 μg/ml
Flow cytometric cell cycle analysis
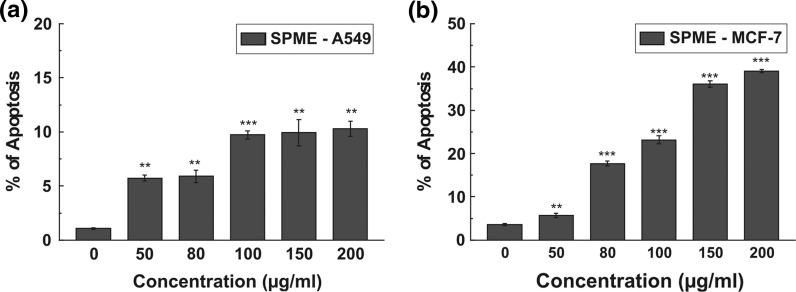
To verify the mechanism by which anticancer effect was achieved, the effect of SPME on cell cycle distribution was studied. As indicated in Fig. 2, SPME increased the number of cells in sub-G1 phase both in the case of A549 and MCF-7 dose dependently, which refers to the cells that underwent apoptosis. This sub-G1 population was quantified as the percentage of apoptosis. These results indicate SPME induced cell death in A549 and MCF-7 cells.
Fig. 2.
Cell cycle distribution was determined in propidium iodide stained samples using flow cytometry. Percentage of cells in sub-G1 was calculated for A549 (a) and MCF-7 (b) cells after treatment with SPME (0–200 μg/ml) for 16 h. All data are expressed as mean ± SD (n = 3). *p < 0.05, **p < 0.01 and ***p < 0.001 versus 0 μg/ml
Apoptosis versus necrosis
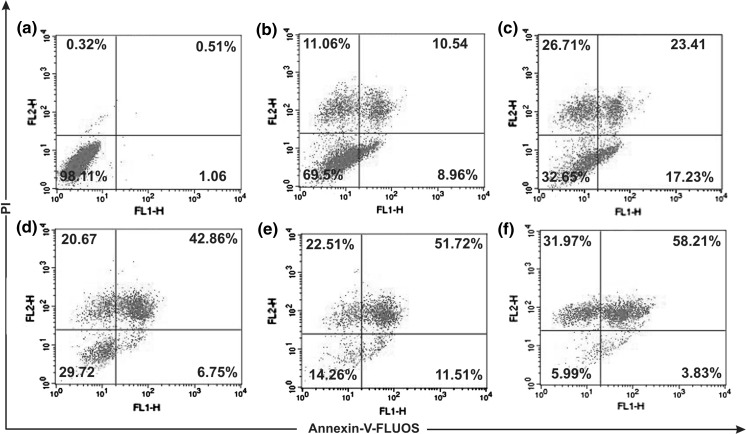
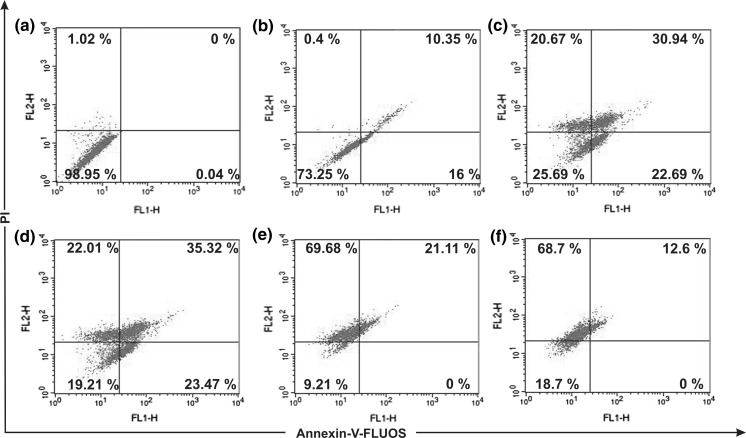
Both cells were treated with SPME (0–200 μg/ml) for 16 h. Figures. 3 and 4 depict the dual parameter dot plots of A549 and MCF-7 cells, respectively. In case of A549 cells, at an SPME treatment of 80 μg/ml, the number of cells in the annexin V (+) quadrant was higher (17.23 %) while higher concentrations resulted in an increasing number of double (+) cells (late apoptotic cells). Whereas, in case of MCF-7 cells, at 100 μg/ml early and late apoptotic cell number was higher compared to lower concentrations but higher concentrations showed a higher number of late apoptotic cells and disrupted cells. These results indicate that SPME effectively induced apoptosis both in A549 and MCF-7 cells at 80 and 100 μg/ml, respectively. So this concentration of SPME was selected for further study to investigate the pathway by which apoptosis had occurred.
Fig. 3.
Flow cytometric plots of annexin-V-FLUOS and propidium iodide stained A549 cells treated for 16 h with different concentrations: Control (a), 50 μg/ml (b), 80 μg/ml (c), 100 μg/ml (d), 150 μg/ml (e), 200 μg/ml (f) of SPME. Numbers in boxes represent % of total cells
Fig. 4.
Flow cytometric plots of annexin-V-FLUOS and propidium iodide staining of MCF-7 cells treated for 16 h with different concentrations: Control (a), 50 μg/ml (b), 80 μg/ml (c), 100 μg/ml (d), 150 μg/ml (e), 200 μg/ml (f) of TBME. Numbers in boxes represents % of total cells
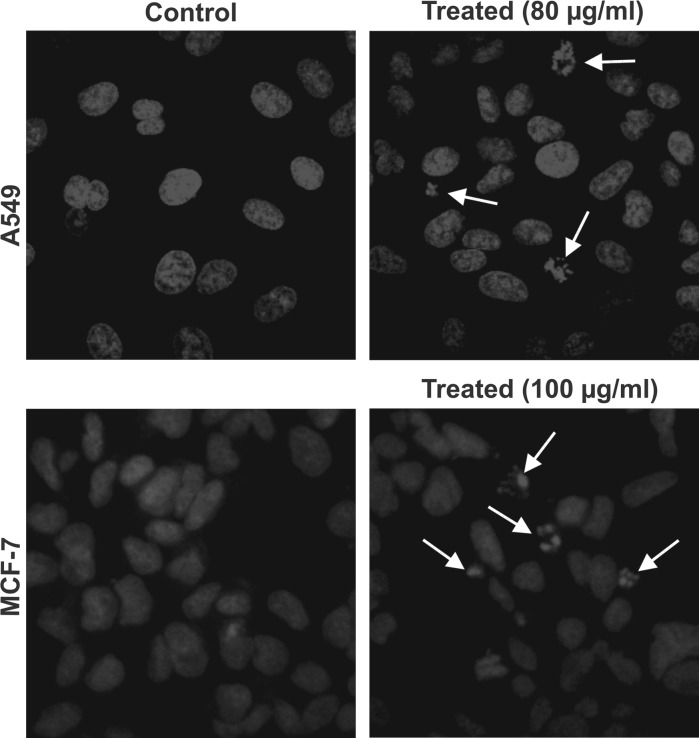
SPME induces DNA fragmentation in A549 and MCF-7 cells
When A549 and MCF-7 cells were treated with SPME at 80 and 100 μg/ml, respectively, for 48 h, appearance of DNA fragmentation and nuclear condensation was observed indicating apoptosis. The capability of SPME to induce morphological changes and apoptosis in both cells was investigated using DAPI stain. Cells with fragmented nuclei are shown in Fig. 5.
Fig. 5.
Detection of DNA fragmentation in 48 h SPME treated A549 and MCF-7 cells. Nuclei were stained with DAPI and observed under confocal microscope. Untreated A549 cells (upper left) and A549 cells treated with 80 μg/ml SPME (upper right); untreated MCF-7 cells (lower left) and MCF-7 cells treated with 100 μg/ml SPME (lower right). The white arrows indicate cells with fragmented nucleus
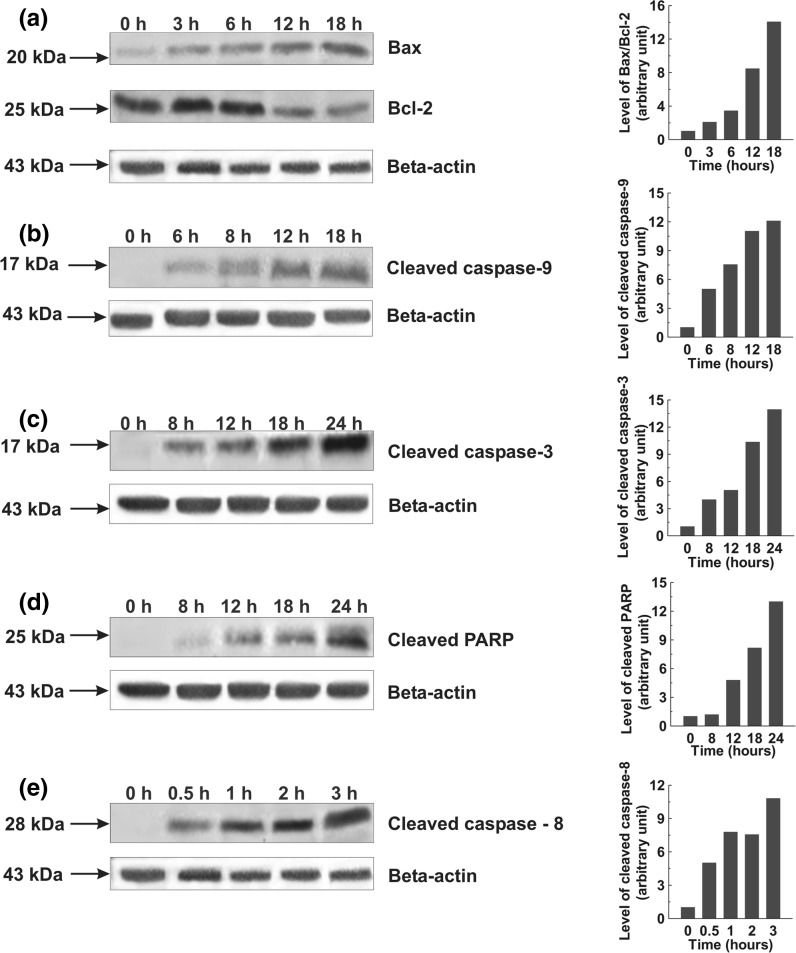
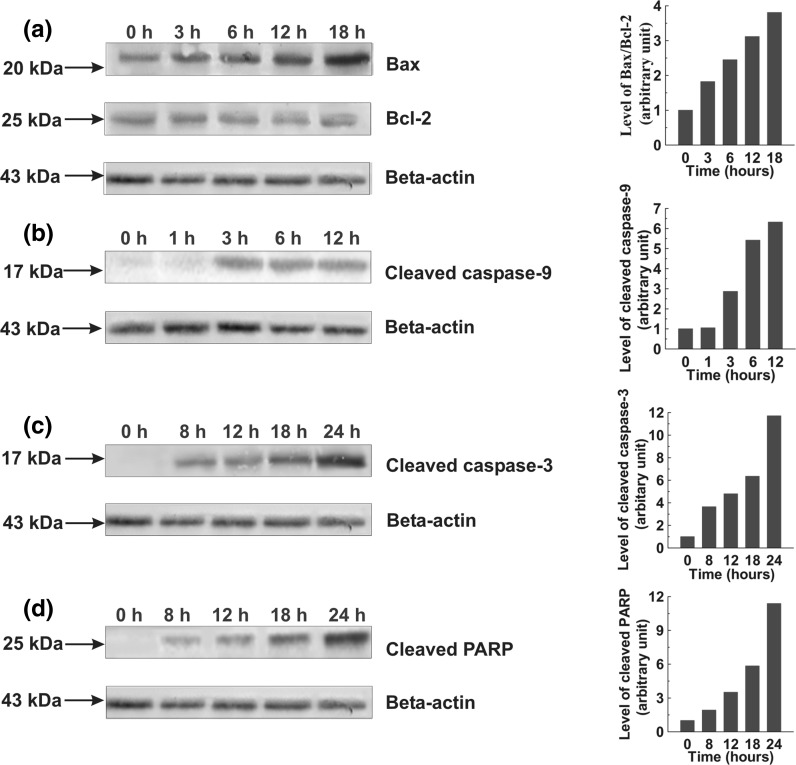
Activation of caspase cascade and PARP cleavage
Western blot was performed on members of caspases and PARP in the cell lysates obtained from SPME treated cells and the results showed increased levels of active caspase-3 and 9 in a time dependent manner in both cells while the appearance of active caspase-8 was only found in A549 cells. The 17 kDa subunit of cleaved caspase-3 was clearly detected after 8 h of treatment in A549 and MCF-7 cells followed by a gradual increase in its level (Figs. 6c, 7c). The cleavage of caspase-8 to its 28 kDa subunit was detected after half an hour of treatment in A549 cells (Fig. 6e). The 17 kDa subunit of cleaved caspase-9 was found increasing with time in both types of cells (Figs. 6b, 7b). Similarly, a role for the cleavage of PARP was shown by the gradual increase in 25 kDa PARP fragment in both types of cells post-treatment with SPME (Figs. 6d, 7d).
Fig. 6.
Whole cell lysates were prepared and resolved followed by western blotting with antibodies specific for the indicated proteins. Graphs adjoining the blots represent the levels of the indicated proteins at given time intervals. Effects of SPME on Bax and Bcl-2 expression; A549 cells were treated with 80 μg/ml SPME for the indicated time intervals (a). Effects of SPME on cleaved caspase-9; A549 cells were treated with 80 μg/ml SPME for the indicated time intervals (b). Effects of SPME on cleaved caspase-3 and PARP; A549 cells were treated with 80 μg/ml SPME for the indicated time intervals (c, d). Effects of SPME on cleaved caspase-8; A549 cells were treated with 80 μg/ml SPME for the indicated time intervals (e)
Fig. 7.
Whole cell lysates were prepared and resolved followed by western blotting with antibodies specific for the indicated proteins. Graphs adjoining the blots represent the levels of the indicated proteins at given time intervals. Effects of SPME on Bax and Bcl-2 expression; MCF-7 cells were treated with 100 μg/ml SPME for the indicated time intervals (a). Effects of SPME on cleaved caspase-9; MCF-7 cells were treated with 100 μg/ml SPME for the indicated time intervals (b). Effects of SPME on cleaved caspase-3 and PARP; MCF-7 cells were treated with 100 μg/ml SPME for the indicated time intervals (c, d)
Alteration in expression of Bcl-2 family proteins
To further explain the molecular mechanism of SPME mediated apoptosis induction, the expression of several Bcl-2 family proteins has been studied. The western blot result showed an enhanced expression of Bax (pro-apoptotic) protein in a time dependent manner with decreased expression of Bcl-2 (anti-apoptotic) protein in A549 cells. On the other hand, the expression of Bax was also found increasing in MCF-7 cell with no change in expression of Bcl-2. Taken together, the results suggest that an increase of the Bax/Bcl-2 ratio might be involved in apoptosis induced by SPME in both type of the cells (Figs. 6a, 7a).
Discussion
In India, the use of medicinal plants and herbal therapy was practised long before recorded history. Remarkably these plants were also found to serve promising anti-oxidants and free radical scavenging agents, which ultimately results in prevention of cancer and autoimmune diseases.
The WST-1 assay showed SPME inhibited the growth of A549 and MCF-7 cells dose dependently. The viability of cells was reduced by 50 % for A549 and 60 % for MCF-7 upon 48 h exposure to SPME dose dependently. By comparison, the cytotoxicity of SPME on human cancer cell lines was found very strong as compared to the normal cells referring to dose response curves and calculated IC50 values. However, this inhibition of growth of both types of cells after treatment with SPME might be resulted from apoptosis, necrosis or cell cycle block (Chan et al. 2010). To further verify whether this effect was from apoptotic induction or cell cycle arrest, flow cytometric analysis of cell cycle and apoptosis was performed. Cell cycle distribution analysis showed that SPME induced cell cycle arrest at sub-G1 phase in a dose dependent manner. In addition, previously it was reported that many cytotoxic agents arrest the cell cycle at G0/G1, S and G2/M phase and then induce apoptosis (Martinez et al. 1987; Torres and Horwitz 1998; Murray 2004; Orren et al. 1997). Apoptosis study using the Annexin-V-FLUOS Staining kit demonstrated that SPME induced early apoptosis significantly 16 h post-treatment in both A549 and MCF-7 cells at 80 and 100 μg/ml, respectively. Internucleosomal DNA fragmentation is the primary hallmark to indicate an early event of apoptosis and it represents a point of no return from the path to cell death (Allen et al. 1997). The observation from DAPI staining also supported that the above dose (80 and 100 μg/ml) of SPME is effective in inducing apoptosis in both types of cells.
As regards the mechanism of apoptosis induction, it is a genetically regulated biological process with two major pathways; namely the death-receptor-induced extrinsic pathway and the mitochondria-apoptosome-mediated intrinsic pathway (Hu and Kavanagh 2003). Bcl-2 family proteins have a vital role in controlling the mitochondrial pathway. The proapoptotic (e.g. Bax, Bak, Bad) and antiapoptotic (e.g. Bcl-2, Bcl-xl, Bcl-l) proteins of the Bcl-2 family may turn on and off apoptosis because of the formation of heterodimers among these proteins (Reed 1997). Therefore, the balance between the expression levels of the protein units (Bcl-2 and Bax) is critical for cell survival and death. Changes in Bax/Bcl-2 ratio and activation of caspase cascade have been reported to be caused by downregulation of Bcl-2 and slight downregulation of Bax (Tian et al. 2008), downregulation of Bcl-2 and upregulation of Bax (Wang et al. 2010). In this investigation, it was found that Bax expression was significantly elevated in SPME treated A549 and MCF-7 cells while Bcl-2 expression remained unchanged in MCF-7 cells and decreased in A549 cells which ultimately resulted in an increase in the Bax/Bcl-2 ratio and activation of the caspase cascade.
Expressed as inactive enzymes, caspases are the family members of cysteine proteases, play a central role in the apoptosis and are activated in a cascade of sequential cleavage reaction (Pastorino et al. 1998), whose activities was assessed for further evidence of apoptosis induction. It is known that caspase-9 is the sole initiator of the intrinsic apoptotic pathway, which is activated in a complex termed the apoptosome by the scaffold protein Apaf-1 and its cofactor cytochrome C (Shi 2002). The present study suggested that caspase-9 was activated in both types of cells which led to the cleavage of caspase-3 to its 17 kDa active subunit. This subunit further triggered the cleavage of PARP and as a whole induced apoptosis. Caspase-8, which is an initiator caspase in case of the extrinsic pathway of apoptosis (Donepudi et al. 2003) was also found activated in A549 cells but not in MCF-7 cells. It was reported that caspase-8 plays a very important role in activation of both the pathways (Anto et al. 2002).
This is the first ever report illuminating the anticancer activity of 70 % methanolic extract of S. pinnata bark against A549 and MCF-7 cell lines, through the induction of apoptosis by altering the expression of Bcl-2 family proteins viz. Bcl-2 and Bax, in other words through the intrinsic pathway. In conclusion, the present report demonstrates that S. pinnata did not exhibit cytotoxicity on a normal lung fibroblast cell line but was effective in inducing apoptosis in a human lung and breast adenocarcinoma cell line. Considering the selectivity of S. pinnata in killing cancer cells it needs to be tested on several other cell lines. It is also important to further characterise the bioactive anticancer compounds present in the same medicinal plant.
Acknowledgments
The authors would like to acknowledge Council of Scientific and Industrial Research, Govt. of India for providing the necessary funds to conduct the study. Acknowledgments are also due to Mr. Ranjit K. Das and Mr. Pradip K. Mallick for their technical assistance.
References
- Allen RT, Hunter WJ, 3rd, Agrawal DK. Morphological and biochemical characterization and analysis of apoptosis. J Pharmacol Toxicol. 1997;37:215–228. doi: 10.1016/S1056-8719(97)00033-6. [DOI] [PubMed] [Google Scholar]
- Anto RJ, Mukhopadhyay A, Denning K, Aggarwal BB. Curcumin (diferuloylmethane) induces apoptosis through activation of caspase-8, BID cleavage and cytochrome c release: its suppression by ectopic expression of Bcl-2 and Bcl-xl. Carcinogenesis. 2002;23:143–150. doi: 10.1093/carcin/23.1.143. [DOI] [PubMed] [Google Scholar]
- Bibitha B, Jisha VK, Salitha CV, Mohan S, Valsa AK. Antibacterial activity of different plant extracts. Indian J Microbiol. 2002;42:361–363. [Google Scholar]
- Chan KT, Meng FY, Li Q, Ho CY, Lam TS, To Y, Lee WH, Li M, Chu KH, Toh M (2010) Cucurbitacin B induces apoptosis and S phase cell cycle arrest in BEL-7402 human hepatocellular carcinoma cells and is effective via oral administration. Cancer Lett 294:118–124. doi:10.1016/j.canlet.2010.01.029 [DOI] [PubMed]
- Christiansen S, Autschbach R. Doxorubicin in experimental and clinical heart failure. Eur J Cardio-Thoracic. 2006;30:611–616. doi: 10.1016/j.ejcts.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Donepudi M, Sweeney AM, Briand C, Grutter MG. Insights into the regulatory mechanism for caspase-8 activation. Mol Cell. 2003;11:543–549. doi: 10.1016/S1097-2765(03)00059-5. [DOI] [PubMed] [Google Scholar]
- Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, Bevers TB, Kavanah MT, Atkins JN, Margolese RG, Runowicz CD, James JM, Ford LG, Wolmark N (2005) Tamoxifen for the Prevention of Breast Cancer: current Status of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer I 97:1652–1662. doi:10.1093/jnci/dji372 [DOI] [PubMed]
- Ghoshal PK, Thakur S. Structural features of the acidic polysaccharide of Spondias pinnata gum exudate. Carbohyd Res. 1981;98:75–83. doi: 10.1016/S0008-6215(00)87143-8. [DOI] [Google Scholar]
- Hazra B, Biswas S, Mandal N. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement Altern Med. 2008;8:63. doi: 10.1186/1472-6882-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazra B, Sarkar R, Mandal N. Spondias pinnata stem bark extract lessens iron overloaded liver toxicity due to hemosiderosis in Swiss albino mice. Ann Hepatol. 2013;12:123–129. [PubMed] [Google Scholar]
- Hu W, Kavanagh JJ. Anticancer therapy targeting the apoptotic pathway. Lancet Oncol. 2003;4:721–729. doi: 10.1016/S1470-2045(03)01277-4. [DOI] [PubMed] [Google Scholar]
- Leksomboon C, Thaveechai N, Kositratana W. Potential of plant extracts for controlling citrus canker of lime. Kasetsart J (Nat Sci) 2001;35:392–396. [Google Scholar]
- Lin YJ, Liu YS, Yeh HH, Cheng TL, Wang LF. Self-assembled poly (ε-caprolactone)-g-chondroitin sulfate copolymers as an intracellular doxorubicin delivery carrier against lung cancer cells. Int J Nanomedicine. 2012;7:4169–4183. doi: 10.2147/IJN.S33602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machana S, Weerapreeyakul N, Barusrux S, Nonpunya A, Sripanidkulchai B, Thitimetharoch T. Cytotoxic and apoptotic effects of six herbal plants against the human hepatocarcinoma (HepG2) cell line. BMC Chin Med. 2011;6:39. doi: 10.1186/1749-8546-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanta RK, Rout SD, Sahu HK. Ethnomedicinal plant resources of Similipal biosphere reserve, Orissa, India. Zoos Print J. 2006;21:2372–2374. doi: 10.11609/JoTT.ZPJ.1435.2372-4. [DOI] [Google Scholar]
- Martínez V, Barberá O, Sánchez-Parareda J, Marco JA. Phenolic and acetylenic metabolites from Artemisia assoana. Phytochem. 1987;26:2619–2624. doi: 10.1016/S0031-9422(00)83891-1. [DOI] [Google Scholar]
- Moreno-Aspitia A, Perez EA. Anthracycline- and/or taxane-resistant breast cancer: results of a literature review to determine the clinical challenges and current treatment trends. Clin Ther. 2009;31:1619–1640. doi: 10.1016/j.clinthera.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Murray AW. Recycling the cell cycle: cyclins revisited. Cell. 2004;116:221–234. doi: 10.1016/S0092-8674(03)01080-8. [DOI] [PubMed] [Google Scholar]
- Orren DK, Petersen LN, Bohr VA. Persistent DNA damage inhibits S-phase and G2 progression, and results in apoptosis. Mol Biol Cell. 1997;8:1129–1142. doi: 10.1091/mbc.8.6.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino JG, Chen ST, Tafani M, Snyder JW, Farber JL. The overexpression of Bax produces cell death upon induction of the mitochondrial permeability transition. J Biol Chem. 1998;273:7770–7775. doi: 10.1074/jbc.273.13.7770. [DOI] [PubMed] [Google Scholar]
- Reed JC. Double identity for proteins of the Bcl-2 family. Nature. 1997;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- Sarkar R, Mandal N. In vitro cytotoxic effect of hydroalcoholic extracts of medicinal plants on Ehrlich’s Ascites Carcinoma. Int J Phytom. 2011;3:370–380. [Google Scholar]
- Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell. 2002;9:459–470. doi: 10.1016/S1097-2765(02)00482-3. [DOI] [PubMed] [Google Scholar]
- Tian Z, Shen J, Moseman AP, Yang Q, Yang J, Xiao P, Wu E, Kohane IS (2008) Dulxanthone A induces cell cycle arrest and apoptosis via up-regulation of p53 through mitochondrial pathway in HepG2 cells. Int J Cancer 122:31–38. doi:10.1002/ijc.23048 [DOI] [PubMed]
- Torres K, Horwitz SB. Mechanisms of taxol-induced cell death are concentration dependent. Cancer Res. 1998;58:3620–3626. [PubMed] [Google Scholar]
- Wang YB, Qin J, Zheng XY, Bai Y, Yang K, Xie LP. Diallyl trisulfide induces Bcl-2 and caspase-3-dependent apoptosis via downregulation of Akt phosphorylation in human T24 bladder cancer cells. Phytomed. 2010;17:363–368. doi: 10.1016/j.phymed.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Wonders KY, Reigle BS. Trastuzumab and doxorubicin-related cardiotoxicity and the cardioprotective role of exercise. Integr Cancer Ther. 2009;8:17–21. doi: 10.1177/1534735408330717. [DOI] [PubMed] [Google Scholar]