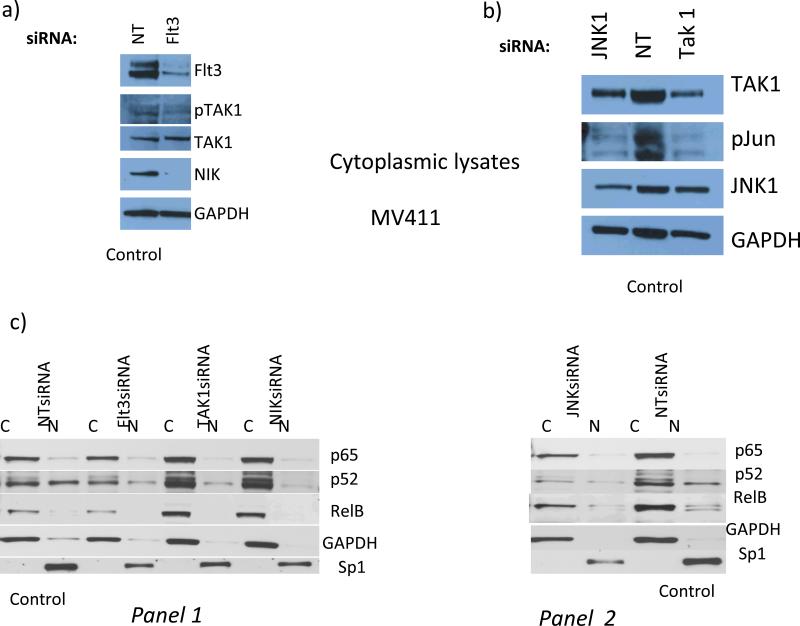
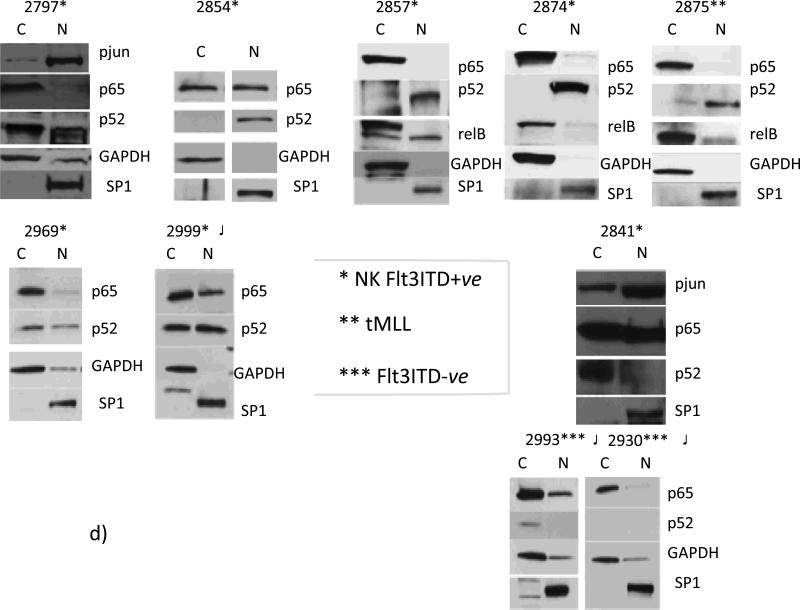
Fig.3. In Flt3ITD+ve AMLs Flt3-to-TAK1 pathway is involved in establishing an effector apparatus involving p52NFκB for DAPK1 repression, whereas primary Flt3ITD-ve AMLs with high DAPK1 expression lack nuclear p52NFκB.
Flt3 knockdown blocked TAK1 activating phosphorylation (78% and 53% by densitometry, respectively) and led to NIK degradation, which inhibits the activation of non- canonical NFκB (panel a). Panels b and c : (b) JNK1 and TAK1 knockdown lead to diminution of c-jun phosphorylation by 100% and 80%, respectively. (c): control MV-4-11 cells (NT siRNA x2), nuclear fraction is characterized by dominant expression levels of p52NFκB and relB, but not p65NFκB. Flt3, TAK1, or NIK knock down lead to diminution of nuclear (N) p52NFκB levels (by 46%, 73%, or 76%, respectively) (panel 1). (Panel 2), JNK1 knockdown led to diminution of p52NFκB levels (by 76%). Flt3 knockdown also reduced overall p52NFκB levels (by 50%) (panel 1). D) Nuclear translocation of p52NFκB is dominant among NKFlt3ITD+ve AML with DAPK1 repression ((#2797, 49%; #2854, 100%; #2857, 70%; #2874, 100%; #2969, 35%; #2999, 50%). Note that ITD+ve sample #2999 was exposed on the same blot with ITD-ve #2993 and 2930, with no nuclear p52NFκB).