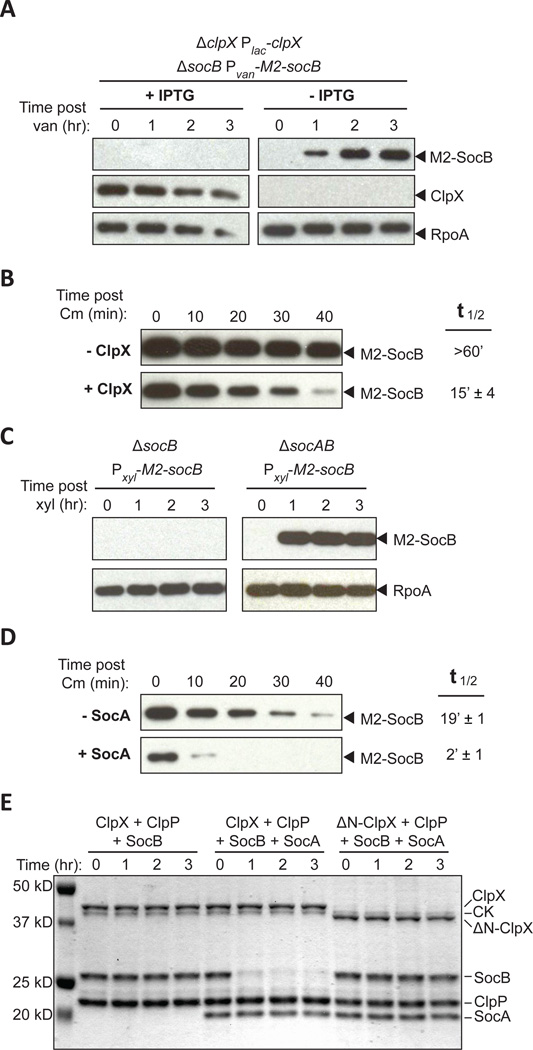
Fig. 2. SocA Promotes SocB Degradation by ClpXP.
(A) Abundance of M2-SocB ± ClpX assessed by immunoblotting. The indicated strain was grown in clpX inducing or repressing conditions for 12 hr and M2-socB expression was induced at time zero. RpoA is a loading control.
(B) Stability of M2-SocB ± ClpX. clpX expression was repressed or induced for 12 hr, and then M2-socB expression was induced for 30 min prior to chloramphenicol (Cm) addition at time zero to shut off protein synthesis. Half-life ± S.E.M. quantified from three replicates (see Fig. S2A).
(C) Abundance of M2-SocB ± SocA assessed by immunoblotting. M2-socB expression was induced at time zero.
(D) Stability of M2-SocB ± SocA. M2-socB expression was induced for 2 hr, and then socA expression was induced for an additional 40 min prior to Cm addition at time zero. Half-life ± S.E.M. quantified from three replicates (see Fig. S2B).
(E) In vitro degradation of SocB by ClpXP ± SocA. Amounts were: 0.5 µM ClpX or ΔN-ClpX, 1 µM ClpP, 5 µM SocB, 5 µM SocA, 32 µg/ml creatine kinase (CK), 16 mM creatine phosphate, and 4 mM ATP. Reaction performed at 4°C.
See also Fig. S2.