Abstract
Women exhibit a nearly twofold increased risk of developing depression and anxiety disorders when compared to men, a fact that has been hypothesized to result in part from increased stress susceptibility. Here, we used the tryptophan hydroxylase-2 R439H knock-in mouse (Tph2KI) and the chronic unpredictable mild stress (CMS) model to examine sex differences in response to congenital 5-HT deficiency and chronic stress. Our results demonstrate that female mice, but not 5-HT-deficient animals, exhibit significantly increased susceptibility to CMS-induced despair-like behavior in the forced swim test. In addition, female 5-HT-deficient mice exhibit anhedonia-like behavior in the sucrose preference test, whereas male 5-HT-deficient animals do not, suggesting that females exhibit increased sensitivity to at least some of the effects of congenital 5-HT deficiency. Although CMS did not reduce cell proliferation in the hippocampus, low levels of brain 5-HT were associated with increased hippocampal cell proliferation, an effect that was predominantly observed in females. Overall, these results highlight the importance of interactions between psychiatric disease risk factors such as sex, chronic stress and congenital 5-HT deficiency in the development of aberrant emotional behavior.
Keywords: serotonin, stress, sex differences, depression, anxiety, hippocampus, neurogenesis, tryptophan hydroxylase – 2, mouse behavior
Introduction
The diathesis-stress hypothesis of psychiatric disease proposes that mental illnesses, such as depression and anxiety disorders, stem from interactions between biological vulnerability factors and exposure to environmental stressors. However, the factors that influence stress susceptibility remain largely unknown. The brain serotonin (5-HT) system has been widely implicated in the etiology of depression and anxiety disorders, largely due to the fact that most antidepressants increase extracellular 5-HT. Support for the 5-HT deficiency theory of depression has also come from clinical studies identifying 5-HT-deficiency-like biomarker alterations in depression patients (Mann et al., 1996; Asberg, 1997) and genetic studies identifying mutations in tryptophan hydroxylase 2 (Tph2), the rate limiting enzyme in brain 5-HT synthesis, in suicide victims and patients with affective disorders, such as major depression (Zill et al., 2004a, b; Zhang et al., 2005; Cichon et al., 2008). However, the factors that regulate susceptibility to the consequences of 5-HT deficiency have not been widely explored, and the effects of congenital 5-HT deficiency on stress vulnerability have not been elucidated.
Women exhibit a nearly two-fold higher incidence of depression and anxiety disorders when compared to men (Breslau et al., 1997; Young and Korszun, 2010), and they have been reported to have increased sensitivity to some of the effects of stress (Weiss et al., 1999) and tryptophan depletion, an experimental model of 5-HT deficiency (Booij et al., 2002). To investigate the impact of sex on susceptibility to chronic stress and congenital 5-HT deficiency, we used the tryptophan hydroxylase - 2 R439H knock-in (Tph2KI) mouse line, which harbors a loss-of-function mutation in the 5-HT synthesis enzyme, tryptophan hydroxylase – 2 (Tph2). Tph2KI mice have been shown to exhibit alterations in several depression- and anxiety-like behaviors and 60-80% reductions in 5-HT in the frontal cortex, hippocampus, striatum and amygdala (Beaulieu et al., 2008; Jacobsen et al., 2012a, b; Sachs et al., 2013a, b).
In the current work, we employed the chronic unpredictable mild stress (CMS) model, which has been previously shown to induce depression- and anxiety-like behavior in rodents (Willner et al., 1987; Surget et al., 2009; Griebel et al., 2002). Here, we examined the responses of Tph2KI mice to CMS to evaluate the combinatorial effects of sex, stress and 5-HT deficiency on depression- and anxiety-like behavior and on hippocampal neurogenesis. Chronic stress has been reported to inhibit adult hippocampal neurogenesis (Gould et al., 1997; Gould et al., 1998; Malberg and Duman, 2003), but the importance of hippocampal neurogenesis for depression- and anxiety-like behavior remains controversial (Petrik et al., 2012). The current study provides preclinical support for the diathesis-stress model of psychiatric disease and sheds new light on the complex interactions that regulate emotional behavior and the factors that influence susceptibility to pathological conditions, such as chronic stress and 5-HT deficiency.
Materials and Methods
Animals
The generation of Tph2KI mice, which are on a mixed background (c57BL6/J – 129S6/SvEvTac), has been described previously (Beaulieu et al., 2008). Age-matched (10-12 weeks old at the start of the experiments) WT and Tph2KI littermates were used for all experiments. Animals used for this study were derived from heterozygous-heterozygous breeding pairs to prevent any potential confounding effects of maternal/paternal behavior. Mice were housed 4-5 per cage except for sucrose preference tests (SPTs), during which they were singly housed. CMS mice (but not controls) were also periodically housed in isolation overnight as part of the CMS paradigm (see below). Control mice were maintained on a 12 h light-dark cycle in a temperature-controlled facility and had ad libitum access to food and water. Experiments were conducted during the light phase. All experiments were performed in accordance with the National Institute of Health's Guide for the Care and Use of Laboratory Animals and were covered by a protocol that had been approved by the Duke University Institutional Animal Care and Use Committee.
Chronic Mild Stress
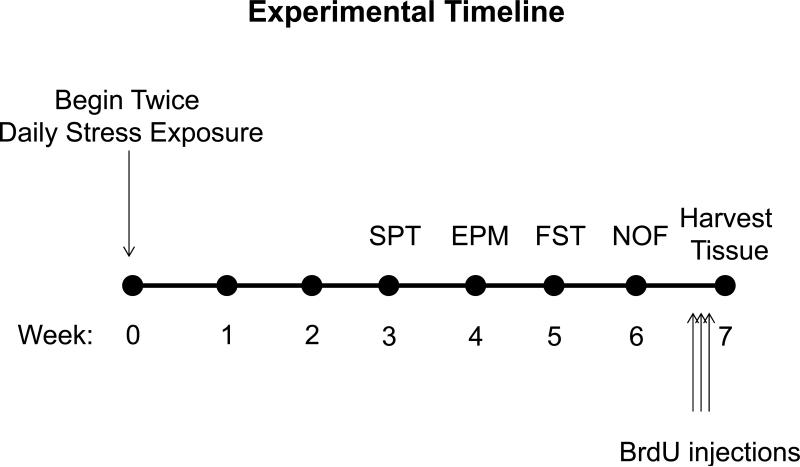
The CMS paradigm involved randomly exposing mice to two of the following stressors each day (except for behavioral testing days, on which mice were not exposed to stressors) for seven weeks (one in the AM – between ~9-11AM, and one in the afternoon between 3-6PM): single housing (overnight), damp bedding (8 h), cage tilting (2 h) , cage shaking (~1 Hz, 1 h), swim stress (10 min), lights on overnight, lights off during the day (3 h) and restraint stress (1 h). Other than for the single housing stressor and for sucrose preference tests, CMS mice were group housed 4-5/cage. The mice were not subjected to any food or water deprivation longer than 1 h. A timeline of stress exposure and behavioral testing can be found in Figure 1.
Figure 1.
A timeline of experiments. SPT = sucrose preference test, EPM = elevated plus maze, FST = forced swim test, NOF = novel open field test.
Behavioral Tests
For the elevated plus maze (EPM), mice were video-recorded and the location and total distance traveled of each mouse over a 5 min period were analyzed using EthoVision, version 7.0 (Noldus, the Netherlands), as described previously (Kumar et al., 2013). The forced swim test (FST) was performed by placing a mouse in a 3 L beaker of water (23 °C) for 6 min and videotaping its behavior. Immobility was scored using EthoVision over the full 6 min. The novel open field (NOF) test was performed by videotaping animals in activity chambers (45 cm × 45 cm, ~125 lux) and analyzing the total distance travelled and the time spent and the distance travelled in the center of the arena over a 20 min period using EthoVision. The EPM, FST and NOF were conducted between 1PM and 6PM. For the SPT, mice were individually housed and given access to two sipper bottles, one with standard drinking water and the other with standard drinking water plus 1% sucrose. The volume of both solutions consumed over an 18 h period (5PM-11AM) was recorded. All bottles were checked for leaks, and any leaking bottles were not included in the final analysis.
Immunohistochemistry
For BrdU immunohistochemistry, mice were injected with BrdU 3 times (i.p., 100 mg/kg, once each at 24, 16 and 4 h prior to ketamine/xylazine euthanasia) and perfused with 10% neutral buffered formalin (NBF). Brains were removed, post-fixed in NBF overnight, cryopreserved in 30% sucrose in PBS for 48 h, embedded in optimal cutting temperature compound (OCT, VWR International, Suwanee, GA), and cut into 20 μm sections using a cryostat. Alternate sections were collected from the first 100 sections through the hippocampus and mounted onto glass slides (a total of 50 sections were collected per animal). Consequently, BrdU incorporation in the hippocampus was only examined from approximately Bregma −0.9 mm to Bregma −2.9 mm, not in the most ventral/temporal/posterior pole of the hippocampus (i.e., from Bregma −2.9 to Bregma ~−3.9).
Sections were dehydrated in two consecutive 5 min washes in xylenes substitute (Sigma, St. Louis, MO, USA) then rehydrated through a series of washes in ethanol (EtOH): 100% EtOH for 10 min, 95% EtOH for 3 min, 85% EtOH for 3 min, then 70% EtOH for 3 min. Slides were washed in PBS for 5 min and subjected to sodium citrate antigen retrieval. Briefly, sections were submerged in sodium citrate antigen retrieval buffer (10 mM sodium citrate and 10 mM citric acid, pH 6.0) and boiled in a microwave for 20 min. Sections were cooled at 4° for 20 min and washed in PBS for 5 min. Sections were then incubated for 1 h in 2 N hydrochloric acid in PBS, washed three times in PBS (5 min each) and then blocked in 5% bovine serum albumin (BSA) in PBS containing 0.1% Triton (PBS-t) for 1 h. After three washes in PBS, sections were incubated overnight in primary antibody diluted in 1% BSA in PBS-t. A rat anti-BrdU primary antibody (OBT0030G, Accurate Chemical Corporation, Westbury, NY, 1:200) was used. Sections were washed three times in PBS and then incubated for 1 h at room temperature with Alexafluor 568-conjugated anti-rat secondary antibody (Life Technologies, Carlsbad, CA) diluted 1:500 in 5% BSA in PBS-t. Sections were then washed three times in PBS and coverslipped using SlowFade Gold Antifade Reagent with DAPI (Life Technologies, Carlsbad, CA).
Non-overlapping images of the subgranular zone from both hemispheres of each section were taken on a fluorescence microscope (Zeiss) by an individual blinded to the genotype and history of stress exposure of the animals. The number of BrdU+ cells was counted by a blinded observer. To examine potential regional differences in hippocampal proliferation, we divided the hippocampus into anterior (~−0.9 to ~−1.9 mm) and posterior (~−1.9 to ~−2.9 mm) regions.
Statistical Analysis
Statistical analyses were performed using JMP Pro 9 software (SAS, Cary, NC). Data were analyzed by two-way or three-way ANOVAs followed by Tukey's post hoc tests, as appropriate. The significance threshold was set at p < 0.05. A table with all of the results from the statistical analyses can be found in the supplemental material.
Results
In the EPM test of anxiety-like behavior, CMS decreased the percent time spent in the open arms (excluding the time spent in the center of the EPM) [F(1,72) = 5.32, p = 0.0239, Fig. 2A], but no significant main effects of sex or genotype, and no significant interaction effects were observed. Similar results were obtained when examining the combined amount of time spent in either the open arms or the center area (not shown). When open arm entries were examined as a percentage of the total number of arm entries, no significant differences were observed between the groups (Fig. 2B).
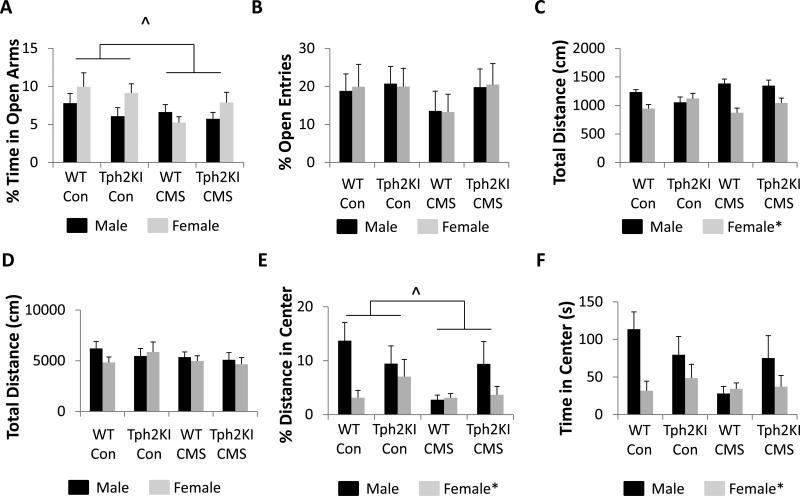
Figure 2.
The effects of CMS on anxiety-like behavior. (A) Open arm time in the EPM. (B) The percent of open arm entries (out of all arm entries). (C)Total distance travelled in the EPM. (D) Total distance travelled in the NOF. (E) Percent distance in the center in the NOF test. (F) Time spent in the center of the NOF. N = 10 per group for A-C and N = 10 per group for D-F. * denotes main effect of sex by three-way ANOVA (p < 0.05). ^ denotes main effect of CMS by three-way ANOVA (p < 0.05). Significant stress by sex and sex by genotype interactions by three-way ANOVA were observed in C. A significant three-way sex by stress by genotype interaction was observed in E.
Female mice exhibited less exploratory locomotion in the EPM than males [F(1,72) = 22.76, p < 0.0001, Fig. 2C], but significant sex × stress [F(1,72) = 7.37, p = 0.0083] and sex × genotype [F(1,72) = 6.7, p = 0.0117] interactions were also observed (Fig. 2C). Post hoc analyses revealed that CMS-exposed WT males were significantly more active than CMS-exposed WT females. When the sexes were examined independently, WT females exhibited decreased activity compared to WT males (p < 0.0001), but no significant sex differences were observed in Tph2KI mice (Fig. 2C).
In the NOF test of motor activity and anxiety-like behavior, no significant differences were observed between any of the groups in total distance traveled (Fig. 2D). When the percent distance traveled in the center of the arena was examined, stress was shown to reduce center distance [F(1,72) = 4.15, p = 0.0452] and females were observed to travel less distance in the center than males [F(1,72) = 6.78, p = 0.0112]. However, a significant three-way interaction between stress, sex and genotype was also observed [F(1,72) = 4.09, p = 0.0468, Fig. 2E]. When the amount of time spent in the center of the arena was examined, female mice were shown to spend significantly less time in the center of the arena than males [F(1,72) = 8.07, p = 0.0058, Fig. 2F], but the three-way interaction was not quite significant (p = 0.065).
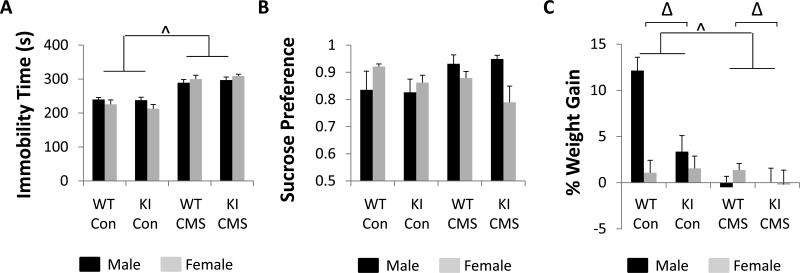
In the FST, CMS increased immobility time in both genotypes and sexes [F(1,71) = 112.61, p < 0.0001], but a sex × stress interaction was also observed [F(1,71) = 5.47, p = 0.022, Fig. 3A]. CMS led to a significantly larger increase in immobility time in females (~39%) than in males (~23%), a finding that reveals an increased susceptibility of females to stress-induced changes in behaviors relevant to depression and/or antidepressant action.
Figure 3.
The effects of CMS on depression-like behavior. (A) Immobility time in the FST. (B) Sucrose preference test. (C) Percent changes in body weight over 7 weeks of CMS. N = 9-10 per group for A, N = 6-9 per group for B and N = 10 per group for C. Δ denotes main effect of genotype by three-way ANOVA (p < 0.05). ^ denotes main effect of CMS by three-way ANOVA (p < 0.05). A significant stress by sex interaction by three-way ANOVA was observed in A and B. A significant three-way interaction between sex, stress and genotype was observed in C, as were significant two-way interactions between sex and stress and stress and genotype (by three-way ANOVA, p < 0.05).
In the SPT, a sex × stress interaction was observed [F(1, 53) = 9.13, p = 0.0039]. Specifically, CMS-exposed females exhibited reduced sucrose preference compared to CMS-exposed males (p = 0.0414), but no CMS-exposed group exhibited significant anhedonia-like behavior compared to controls (Fig. 3B). When the sexes were evaluated independently, CMS led to an unexpected increase in sucrose consumption and preference in male mice [F(1,20) = 6.8869, p = 0.0162]. In females, CMS did not have a significant effect on sucrose preference, but 5-HT deficiency significantly reduced sucrose preference regardless of stress condition [F(1, 33) = 4.4164, p = 0.0433], thus demonstrating that females exhibit increased sensitivity to a subset of depression-like behaviors induced by 5-HT deficiency.
When changes in body weight were examined, a three-way sex × stress × genotype interaction was observed [F(1, 72) = 3.9836, p = 0.0497, Fig. 3C]. When the sexes were analyzed separately, CMS was shown to decrease weight gain in both females [F(1,36) = 4.4851, p = 0.0412] and males [F(1,36) = 31.3311, p < 0.0001]. In males, WT mice gained more weight than Tph2KI animals [F(1,36) = 8.4571, p = 0.0062], but a significant genotype × stress interaction was also observed [F(1,36) = 10.3753, p = 0.0027], and control WT males exhibited significantly more weight gain than any other group (p's < 0.0003). No significant effects of 5-HT deficiency on body weight were observed at baseline or at any time point following stress.
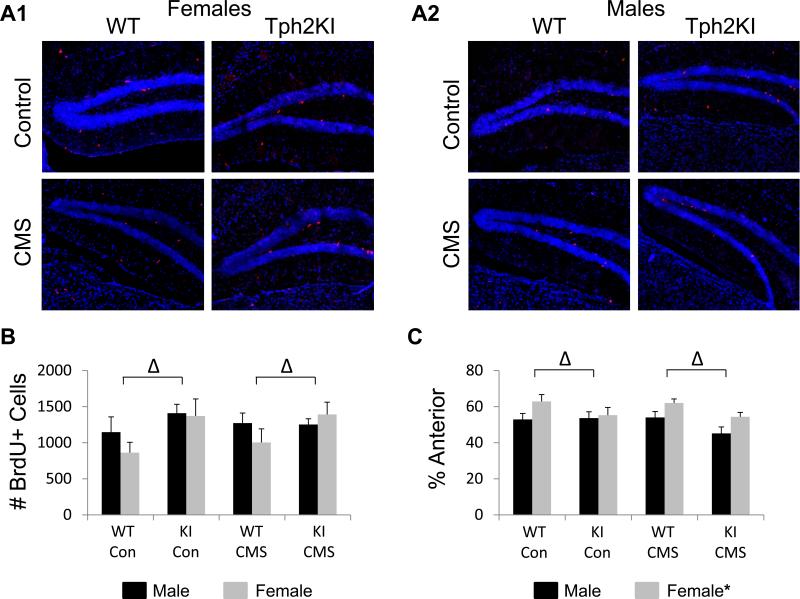
In our hands, CMS failed to reduce cell proliferation in the hippocampus (Fig. 4A,B), but 5-HT deficiency led to an overall increase in hippocampal cell proliferation [F(1, 68) = 6.2504, p = 0.0148, Fig. 4A,B]. Although no significant interaction effects were observed, the increased hippocampal proliferation of Tph2KI animals occurred primarily in females (~50%), not in males (<10%). When regional differences in hippocampal proliferation were compared, females were observed to exhibit an increased proportion of proliferation in the anterior portion (Bregma ~−0.9 to −1.9) of the hippocampus when compared to males [F(1, 68) = 10.3558, p = 0.002, Fig. 4C]. In addition, 5-HT deficiency decreased the relative proportion of proliferation in the anterior hippocampus compared to the posterior portion (Bregma ~−1.9 to −2.9) of the hippocampus [F(1,68) = 6.8353, p = 0.01, Fig. 4C].
Figure 4.
The effects of CMS, sex and 5-HT deficiency on cell proliferation in the hippocampus. Representative images from WT control, WT CMS, Tph2KI control and Tph2KI CMS females (A1) and males (A2). BrdU quantification is shown for both genotypes, sexes and stress conditions (B). The percentage of proliferating cells observed in the ventral/anterior hippocampus is shown for both genotypes, sexes and stress conditions (C). * denotes a main effect of sex and Δ denotes a main effect of genotype by three-way ANOVA (p < 0.05).
Discussion
The biological basis of the increased incidence of depression and anxiety disorders in women remains unknown, but females have been reported to exhibit increased susceptibility to the effects of stress (Weiss et al., 1999) and low levels of 5-HT (Booij et al., 2002), two putative risk factors for psychiatric disease. Although relatively few studies have investigated sex differences in stress susceptibility in rodents, sex differences have been reported following stress alone (Dalla et al., 2005) and following stress in combination with neurotrophin deficiency (Autry et al., 2009) or lack of the 5-HT transporter (Joeyen-Waldorf et al., 2009). Our results confirm the importance of sex as a determinant of stress susceptibility and reveal that sex also increases vulnerability to congenital 5-HT deficiency. Surprisingly, our data identify no significant effects of congenital 5-HT deficiency on susceptibility to CMS. In contrast, we have previously shown that 5-HT deficiency impacts behavioral responses to mild early life stress (Sachs et al., 2013b). Thus, we propose that the effects of 5-HT deficiency on stress sensitivity likely depend on the type, timing and intensity of stress exposure.
Our data demonstrate that 5-HT deficiency decreases sucrose preference only in females, which is consistent with clinical reports that females are more susceptible to the mood-depressing effects of 5-HT depletion (Booij et al., 2002). Similarly, our results demonstrate that females are also more susceptible to the behavioral despair-inducing effects of CMS in the FST. In addition, female mice were observed to exhibit an overall increase in anxiety-like behavior in the NOF test. The lack of a CMS-induced increase in anxiety-like behavior in the NOF in females could potentially be interpreted to suggest that females exhibit ‘resilience’ to stress, which would be inconsistent with our other results. However, given that female mice exhibited a baseline increase in anxiety-like behavior in this test, we hypothesize that their lack of further response simply reflects a floor/ceiling effect. Together, our behavioral findings support the hypothesis that at least some of the increased incidence of depression and anxiety disorders in females is due to biological sex differences rather than gender differences (i.e., societal roles).
In the current work, CMS did not significantly reduce sucrose preference, which conflicts with the majority of previous reports in rats (Willner et al., 1987). However, several other studies have also reported increased sucrose preference following CMS (reviewed in Willner, 2005), and the reasons for these ‘anomalous’ responses to CMS remain unclear. In contrast to the original CMS procedure in rats described by Willner et al., the current study did not employ food or water deprivation. In addition, the rats used in the Willner studies were single housed throughout most of the CMS procedure, except for when they were pair housed as part of the stress exposure. In contrast, the mice used in the current study were group housed except for occasional bouts of isolation housing as part of the stress exposure. Thus, given the many differences between the paradigms, it is not unexpected that differing behavioral profiles were observed. Nonetheless, the CMS procedure employed here did produce depression- and anxiety-like phenotypes in the EPM, NOF and FST.
The use of forced swimming as a stressor in the current study may have influenced the subsequent performance of mice in the FST, but future research would be required to evaluate the specific contribution of any individual stressor (e.g., forced swimming or circadian disruptions) to any of the behavioral phenotypes observed. In addition, future research will be required to evaluate the effects of the specific CMS paradigm employed here on other mouse strains, as strain is known to affect stress-induced phenotypes in mice (Polthion et al., 2004), and the phenotypes observed in the current study may be specific to the mixed genetic background of the mice examined here.
The importance of hippocampal neurogenesis in depression- and anxiety-like behavior and stress responses remains highly controversial (Petrik et al., 2012). Several studies have reported that chronic stress reduces adult hippocampal neurogenesis (Gould et al., 1997, 1998), a finding that we did not observe here. However, similar to the current findings, several other studies have documented significant behavioral responses to stress in the absence of stress-induced changes in hippocampal neurogenesis (Airan et al., 2007; Hanson et al., 2011). Although the reasons underlying these differences remain obscure, at least one previous study has reported that chronic stress preferentially inhibits cell proliferation and neurogenesis in the ‘ventral’ dentate gyrus (Tanti et al., 2012), and unfortunately the extreme ‘ventral’ pole of the hippocampus was not examined in the current study.
Although our current results did not reveal any significant effects of stress on hippocampal neurogenesis, they do reveal that sex and 5-HT deficiency exhibit a differential impact on cell proliferation along the anterior-posterior dimensions of the hippocampus (at least within the anterior/dorsal/septal two-thirds of the structure). Prior work has shown that the rates of neurogenesis differ along the septo-temporal axis of the hippocampus (Snyder et al., 2009, 2011), but the factors that lead to these regional differences in hippocampal neurogenesis remain poorly defined. Our results identify sex and 5-HT deficiency as two factors regulating regional differences in hippocampal neurogenesis and highlight the possibility that alterations in the regional balance of hippocampal neurogenesis may have important implications for sex- and 5-HT-deficiency-induced behavioral changes.
Previous work has revealed that 5-HT deficiency leads to a significant increase in the survival of neural progenitor cells in 12-week-old male mice, but does not affect their proliferation (Diaz et al., 2013; Sachs et al., 2013a). Our current data reveal that 5-HT deficiency can also affect hippocampal cell proliferation, at least in older (~19 weeks) female mice. Notably, we did not explore the survival or differentiation of progenitor cells in the hippocampus following CMS in the current study, and thus our data do not rule out a role for stress-induced changes in hippocampal neurogenesis in mediating stress-induced behavioral alterations. Future research will be required to determine the combinatorial effects of sex, stress and 5-HT deficiency on cell survival and neurogenesis throughout the entire hippocampus (including the ventral portions). Similarly, it will be important to evaluate the effects of these factors on brain regions other than the hippocampus, as it is likely that other brain regions also play key roles in mediating the effects of sex, 5-HT deficiency and stress on animal behavior.
Overall, our results demonstrate that females exhibit increased sensitivity to depression-like phenotypes induced by stress and 5-HT deficiency. In contrast, female mice were not observed to exhibit increased susceptibility to stress-induced anxiety-like behavior, although females were characterized by a baseline increase in anxiety-like behavior in the NOF. An improved understanding of the precise behavioral domains that are impacted by psychiatric disease risk factors, such as sex, stress and 5-HT deficiency, may provide further insight into sex differences in the incidence and manifestations of neuropsychiatric illness.
Supplementary Material
Acknowledgements
This work was supported in part by grants from the National Institutes of Health (MH79201 and MH60451) to MGC. BDS was the recipient of a Minority Supplement award from the National Institutes of Health (MH79201-03S1) and is currently the recipient of an NRSA postdoctoral fellowship (F32- MH093092). The authors thank Wendy Roberts, Akshita Iyer, Ha Tran and Sean Berkowitz for technical assistance and Dr. Kafui Dzirasa for facility and equipment access.
Role of the funding source
This work was supported in part by grants from the National Institutes of Health (MH79201 and MH60451) to MGC. These sources had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
BDS designed the study, performed the experiments, analyzed the data and wrote the paper. JN performed experiments, analyzed data and helped revise the paper. MGC designed the study, analyzed data and revised the paper. All authors have contributed to and approved the final manuscript.
Conflict of Interest
The authors declare no competing financial interests.
References
- Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–823. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- Asberg M. Neurotransmitters and suicidal behavior. The evidence from cerebrospinal fluid studies. Ann N Y Acad Sci. 1997;836:158–181. doi: 10.1111/j.1749-6632.1997.tb52359.x. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Cheng P, Monteggia LM. Gender-specific impact of brain-derived neurotrophic factor signaling on stress-induced depression-like behavior. Biol Psychiatry. 2009;66:84–90. doi: 10.1016/j.biopsych.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Zhang X, Rodriguiz RM, Sotnikova TD, Cools MJ, Wetsel WC, Gainetdinov RR, Caron MG. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc Natl Acad Sci U S A. 2008;105:1333–1338. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij L, Van der Does W, Benkelfat C, Bremner JD, Cowen PJ, Fava M, Gillin C, Leyton M, Moore P, Smith KA, Van der Kloot WA. Predictors of mood response to acute tryptophan depletion. A reanalysis. Neuropsychopharmacology. 2002;27:852–861. doi: 10.1016/S0893-133X(02)00361-5. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P, Peterson EL, Schultz LR. Sex differences in posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54:1044–1048. doi: 10.1001/archpsyc.1997.01830230082012. [DOI] [PubMed] [Google Scholar]
- Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219–229. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- Cichon S, et al. Brain-specific tryptophan hydroxylase 2 (TPH2): a functional Pro206Ser substitution and variation in the 5′-region are associated with bipolar affective disorder. Hum Mol Genet. 2008;17:87–97. doi: 10.1093/hmg/ddm286. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Drossopoulou G, Xagoraris M, Kokras N, Sfikakis A, Papadopoulou-Daifoti Z. Chronic mild stress impact: are females more vulnerable? Neuroscience. 2005;135:703–714. doi: 10.1016/j.neuroscience.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Diaz SL, Narboux-Neme N, Trowbridge S, Scotto-Lomassese S, Kleine Borgmann FB, Jessberger S, Giros B, Maroteaux L, Deneris E, Gaspar P. Paradoxical increase in survival of newborn neurons in the dentate gyrus of mice with constitutive depletion of serotonin. Eur J Neurosci. 2013 doi: 10.1111/ejn.12297. [DOI] [PubMed] [Google Scholar]
- Fernandez SP, Gaspar P. Investigating anxiety and depressive-like phenotypes in genetic mouse models of serotonin depletion. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.08.049. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci U S A. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Serradeil-Le Gal C, Wagnon J, Pascal M, Scatton B, Maffrand JP, Soubrie P. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc Natl Acad Sci U S A. 2002;99:6370–6375. doi: 10.1073/pnas.092012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson ND, Owens MJ, Boss-Williams KA, Weiss JM, Nemeroff CB. Several stressors fail to reduce adult hippocampal neurogenesis. Psychoneuroendocrinology. 2011;36:1520–1529. doi: 10.1016/j.psyneuen.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Jacobsen JP, Medvedev IO, Caron MG. The 5-HT deficiency theory of depression: perspectives from a naturalistic 5-HT deficiency model, the tryptophan hydroxylase 2Arg439His knockin mouse. Philos Trans R Soc Lond B Biol Sci. 2012a;367:2444–2459. doi: 10.1098/rstb.2012.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen JP, Siesser WB, Sachs BD, Peterson S, Cools MJ, Setola V, Folgering JH, Flik G, Caron MG. Deficient serotonin neurotransmission and depression-like serotonin biomarker alterations in tryptophan hydroxylase 2 (Tph2) loss-of-function mice. Mol Psychiatry. 2012b;17:694–704. doi: 10.1038/mp.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joeyen-Waldorf J, Edgar N, Sibille E. The roles of sex and serotonin transporter levels in age- and stress-related emotionality in mice. Brain research. 2009;1286:84–93. doi: 10.1016/j.brainres.2009.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Black SJ, Hultman R, Szabo ST, DeMaio KD, Du J, Katz BM, Feng G, Covington HE, 3rd, Dzirasa K. Cortical control of affective networks. J Neurosci. 2013;33:1116–1129. doi: 10.1523/JNEUROSCI.0092-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Donovan MH, DeCarolis NA, Farnbauch LA, Malhotra S, Berton O, Nestler EJ, Krishnan V, Eisch AJ. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc Natl Acad Sci U S A. 2010;107:4436–4441. doi: 10.1073/pnas.0910072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Maejima T, Wyler SC, Casadesus G, Herlitze S, Deneris ES. Pet-1 is required across different stages of life to regulate serotonergic function. Nat Neurosci. 2010;13:1190–1198. doi: 10.1038/nn.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Malone KM, Psych MR, Sweeney JA, Brown RP, Linnoila M, Stanley B, Stanley M. Attempted suicide characteristics and cerebrospinal fluid amine metabolites in depressed inpatients. Neuropsychopharmacology. 1996;15:576–586. doi: 10.1016/S0893-133X(96)00102-9. [DOI] [PubMed] [Google Scholar]
- Petrik D, Lagace DC, Eisch AJ. The neurogenesis hypothesis of affective and anxiety disorders: are we mistaking the scaffolding for the building? Neuropharmacology. 2012;62:21–34. doi: 10.1016/j.neuropharm.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothion S, Bizot JC, Trovero F, Belzung C. Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav Brain Res. 2004;155:135–146. doi: 10.1016/j.bbr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Sachs BD, Jacobsen JP, Thomas TL, Siesser WB, Roberts WL, Caron MG. The effects of congenital brain serotonin deficiency on responses to chronic fluoxetine. Transl Psychiatry. 2013a;3:e291. doi: 10.1038/tp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs BD, Rodriguiz RM, Siesser WB, Kenan A, Royer EL, Jacobsen JP, Wetsel WC, Caron MG. The effects of brain serotonin deficiency on behavioural disinhibition and anxiety-like behaviour following mild early life stress. Int J Neuropsychopharmacol. 2013b:1–14. doi: 10.1017/S1461145713000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Radik R, Wojtowicz JM, Cameron HA. Anatomical gradients of adult neurogenesis and activity: young neurons in the ventral dentate gyrus are activated by water maze training. Hippocampus. 2009;19:360–370. doi: 10.1002/hipo.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Ramchand P, Rabbett S, Radik R, Wojtowicz JM, Cameron HA. Septo-temporal gradients of neurogenesis and activity in 13-month-old rats. Neurobiol Aging. 2011;32:1149–1156. doi: 10.1016/j.neurobiolaging.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surget A, Wang Y, Leman S, Ibarguen-Vargas Y, Edgar N, Griebel G, Belzung C, Sibille E. Corticolimbic transcriptome changes are state-dependent and region-specific in a rodent model of depression and of antidepressant reversal. Neuropsychopharmacology. 2009;34:1363–1380. doi: 10.1038/npp.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanti A, Rainer Q, Minier F, Surget A, Belzung C. Differential environmental regulation of neurogenesis along the septo-temporal axis of the hippocampus. Neuropharmacology. 2012;63:374–384. doi: 10.1016/j.neuropharm.2012.04.022. [DOI] [PubMed] [Google Scholar]
- Weiss EL, Longhurst JG, Mazure CM. Childhood sexual abuse as a risk factor for depression in women: psychosocial and neurobiological correlates. Am J Psychiatry. 1999;156:816–828. doi: 10.1176/ajp.156.6.816. [DOI] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Young E, Korszun A. Sex, trauma, stress hormones and depression. Molecular psychiatry. 2010;15:23–28. doi: 10.1038/mp.2009.94. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gainetdinov RR, Beaulieu JM, Sotnikova TD, Burch LH, Williams RB, Schwartz DA, Krishnan KR, Caron MG. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 2005;45:11–16. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Zill P, Buttner A, Eisenmenger W, Moller HJ, Bondy B, Ackenheil M. Single nucleotide polymorphism and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene in suicide victims. Biol Psychiatry. 2004a;56:581–586. doi: 10.1016/j.biopsych.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Zill P, Baghai TC, Zwanzger P, Schule C, Eser D, Rupprecht R, Moller HJ, Bondy B, Ackenheil M. SNP and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene provide evidence for association with major depression. Mol Psychiatry. 2004b;9:1030–1036. doi: 10.1038/sj.mp.4001525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.