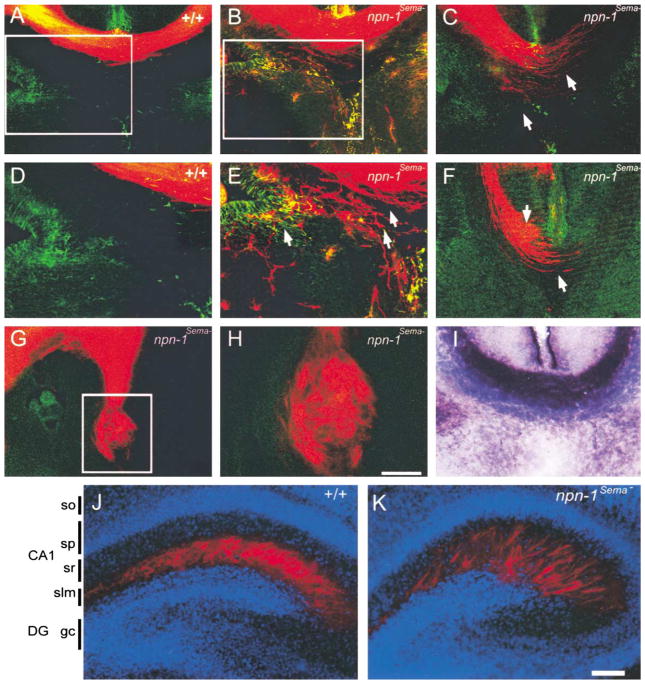
Figure 4. Axonal Projection Defects in the Corpus Callosum and Hippocampus in npn-1Sema− Mice.
(A–H) DiI labeling at E17.5 was used to investigate development of the corpus callosum in wild-type (A and D) and npn-1Sema− mice (B, C, and E–H). The corpus callosum developed normally in all wild-type littermate embryos analyzed (n = 10), whereas in all npn-1Sema− embryos examined (n = 10), callosal axons (red) displayed varying degrees of defasciculation from more mild phenotypes (arrows in [E]) to more severe phenotypes (arrows in [C] and [F]). In the most extreme cases, callosal axons formed Probst bundles (G and H). Sections were double labeled with GFAP immunohistochemistry to label midline glial structures (A–H). In some cases, axons grew through the glial wedge (green labeling in [E]) and into the septum.
(I) AP-Sema3A section binding reveals a high level of Sema3A binding to axons of the E17.5 corpus callosum in wild-type mice. (D), (E), and (H) are high-magnification views of the boxed regions in (A), (B), and (G), respectively.
(J–K) Sema-Npn-1 signaling is crucial for layer-specific targeting of entorhinohippocampal projections. Coronal sections of brains of P2 wild-type (J) and npn-1Sema− littermate mice (K) showing DiI-labeled (red) entorhinohippocampal axons. The sections were counterstained with bis-benzimide (blue) to reveal the hippocampal architecture. In wild-type mice, entorhinal fibers (red) are restricted to the stratum lacunosum moleculare (slm) (J). In contrast, entorhinal fibers of npn-1Sema− mice are found in the slm layer and also ectopic layers such as the stratum radiatum (sr) of the CA1 field (K). DG, dentate gyrus; slm, stratum lacunosum moleculare; sp, stratum pyramidale; sr, statum radiatum. Scale bars: (A–C, F, and I), 150 μm; (G), 200 μm; (H), 60 μm; (J and K), 100 μm.