Significance
β-Catenin is an important versatile protein in most types of cells and its activity is regulated by differential phosphorylations and, hence, affects cellular functions. In this study, we defined a unique mechanism by which different modes of heterocellular interaction between vascular endothelial cells (ECs) and smooth muscle cells (SMCs) play differential roles in regulating EC β-catenin phosphorylations and functions. Direct physical interaction of SMCs to ECs causes their β-catenin Tyr142-phosphorylation via connexin 43/Fer to induce EC inflammation. In contrast, paracrine interaction of SMCs to ECs induces Ser45/Thr41-phosphorylation of VE-cadherin–associated β-catenin in ECs to cause their barrier dysfunction, with increased permeability. Our findings suggest that different phosphorylated forms of β-catenin play important roles in modulating vascular pathophysiology through different heterocellular interactions.
Keywords: endothelial permeability, inflammation
Abstract
β-Catenin phosphorylation plays important roles in modulating its functions, but the effects of different phosphorylated forms of β-catenin in response to heterocellular interaction are unclear. Here we investigated whether distinct modes of phosphorylation on β-catenin could be triggered through heterocellular interactions between endothelial cells (ECs) and smooth muscle cells (SMCs), and the consequent modulation of EC functions. ECs were cocultured with SMCs to initiate direct contact and paracrine interaction. EC–SMC coculture induced EC β-catenin phosphorylations simultaneously at tyrosine 142 (Tyr142) and serine 45/threonine 41 (Ser45/Thr41) at the cytoplasm/nuclei and the membrane, respectively. Treating ECs with SMC-conditional medium induced β-catenin phosphorylation only at Ser45/Thr41. These findings indicate that different phosphorylation effects of EC–SMC coculture were induced through heterocellular direct contact and paracrine effects, respectively. Using specific blocking peptides, antagonists, and siRNAs, we found that the β-catenin Tyr142-phosphorylation was mediated by connexin 43/Fer and that the β-catenin Ser45/Thr41-phosphorylation was mediated by SMC-released bone morphogenetic proteins through VE-cadherin and bone morphogenetic protein receptor-II/Smad5. Transfecting ECs with β-catenin-Tyr142 or -Ser45 mutants showed that these two phosphorylated forms of β-catenin modulate differential EC function: The Tyr142-phosphorylated β-catenin stimulates vascular cell-adhesion molecule-1 expression to increase EC-monocytic adhesion, but the Ser45/Thr41-phosphorylated β-catenin attenuates VE-cadherin–dependent junction structures to increase EC permeability. Our findings provide new insights into the understanding of regulatory complexities of distinct modes of β-catenin phosphorylations under EC–SMC interactions and suggest that different phosphorylated forms of β-catenin play important roles in modulating vascular pathophysiology through different heterocellular interactions.
It is known that β-catenin is a major component of cell junctions and a gene transcription factor that plays important roles in regulating cell functions (1). A well-known regulatory mechanism of β-catenin is through the N-terminal serial phosphorylations at serine 45 (Ser45) and then threonine 41 (Thr41)/Ser33/37, which consequently initiate the self-degradation of β-catenin. However, recent evidence indicates that the effects of different phosphorylated modes of β-catenin, including tyrosine (Tyr) 142/654 and Ser-45, on its activity regulations are more complex than originally understood (1, 2). For example, β-catenin Ser45-phosphorylation does not necessarily lead to its degradation, but instead modulates cell migration in tumor cells (2); β-catenin Tyr142/654-phosphorylation by membrane receptor tyrosine kinases (RTKs) or cytosolic Fyn/Fer tyrosine kinases increases its transcription cofactor activity to regulate target gene activations in epithelial and tumor cells (1, 3). In addition, β-catenin activity has been shown to be coordinated by many growth factors, such as, bone morphogenetic proteins (BMPs) and their Smad signaling, to modulate cell functions (4). Although the influences of distinct phosphorylated forms of β-catenin on cell functions have been studied, the detailed mechanisms that regulate the differential phosphorylations on β-catenin and hence cell functions remain unclear.
Heterocellular interactions between vascular endothelial cells (ECs) and smooth muscle cells (SMCs) play critical roles in regulating many vascular functions and diseases (5). Within the vessel wall, coordinated vascular EC and SMC functions are achieved by different mechanisms of intercellular signaling, including direct physical contact and paracrine interaction (6). Accumulating evidence indicates that a multitude of paracrine mediators regulate vascular functions through β-catenin, which plays important roles in vascular biology (4). EC–SMC direct contact via connexins (Cxs)/gap junctions and cadherin/adherens junctions has been demonstrated to play important roles in modulating vascular biology and pathobiology, and β-catenin has been shown to regulate cell–cell interaction through both types of junctions (7–9). It is not clearly understood whether direct and paracrine interactions between ECs and SMCs play differential roles in modulating EC β-catenin phosphorylation, and hence differential functions in ECs.
In the present study, we investigated the roles of different EC–SMC heterocellular interactions in regulating distinct modes of phosphorylation on β-catenin and how these different phosphorylated forms of β-catenin modulate EC functions. We found that SMCs stimulate EC β-catenin phosphorylations at both (i) Tyr142 in the cytoplasm and nuclei through Cx43/Fer via EC–SMC direct contact, and (ii) Ser45/Thr41 at the membrane through BMP receptor-II (BMPRII)/Smad5 and VE-cadherin and paracrine release of BMPs. Both of these two types of phosphorylation on β-catenin result in inflammatory responses of ECs, but with different functional consequences: Tyr142 phosphorylation stimulates vascular cell adhesion molecule-1 (VCAM-1) expression to increase EC-monocytic adhesion, whereas Ser45/Thr41 phosphorylation attenuates the VE-cadherin–dependent EC–EC junction to increase EC permeability. Our findings provide new insights into the understanding of the regulatory complexities and importance of distinct modes of β-catenin phosphorylations under heterocellular interactions and the consequent modulation of cell functions.
Results
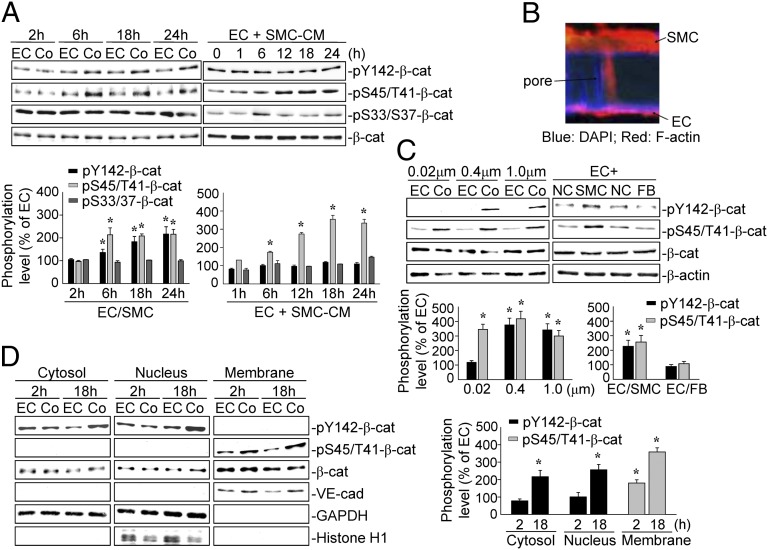
Direct Contact and Paracrine Interaction Between ECs and SMCs Induce Differential Phosphorylations of β-Catenin at Different Cellular Compartments in ECs.
Human umbilical vein ECs (referred to as ECs) and artery SMCs (referred to as SMCs) were used for all experiments except those involving the transfection of cells with β-catenin plasmids, in which bovine aortic ECs (BAECs) and SMCs (BASMCs) were used. ECs and SMCs were cocultured on opposite sides of a 1.0-μm pore-size membrane to form an EC/SMC coculture. The coculture resulted in EC β-catenin phosphorylations at both Tyr142 and Ser45/Thr41 after 6 h and persisted until 24 h, compared with the monocultured ECs (Fig. 1A). Immunostaining with a specific antibody against F-actin showed that SMCs can penetrate through the pore channels to reach the EC side after coculture for 24 h (Fig. 1B). The penetration of a SMC through a 1.0-μm pore to make contact with an EC is shown in Fig. S1. In contrast, SMCs in EC–SMC coculture with a 0.02-μm pore-size membrane cannot penetrate into the pores (Fig. S2). Treatment of ECs with SMC-conditioned medium (CM) induced an increase (within 6 h) in β-catenin Ser45/Thr41-phosphorylation that persisted at 24 h (Fig. 1A). However, the SMC-CM did not induce β-catenin Tyr142-phosphorylation in ECs. The β-catenin Ser45/Thr41-phosphorylation in ECs induced by coculture and by SMC-CM treatment was not accompanied by an increase in the degradation marker β-catenin Ser33/37-phosphorylation or a decrease in β-catenin protein level (Fig. 1A). To further determine the critical roles of SMCs in EC β-catenin phosphorylations, ECs were cocultured with SMCs on 0.02-, 0.4-, and 1.0-μm pore-size membranes for 18 h. Only coculturing on 0.4- and 1.0-μm pore-size membranes resulted in EC β-catenin Tyr142-phosphorylation, but all three types of membranes resulted in β-catenin Ser45/Thr41-phosphorylation (Fig. 1C). Coculturing ECs with fibroblasts did not induce any mode of EC β-catenin phosphorylation found with the EC–SMC coculture (Fig. 1C). The β-catenin Tyr142-phosphorylation induced by EC–SMC coculture was found in the cytosolic and nuclear compartments, whereas the Ser45/Thr41-phosphorylation was observed at the membrane (Fig. 1D). These results indicate that β-catenin Tyr142- and Ser45/Thr41-phosphorylations in different cellular compartments of ECs induced by EC–SMC coculture were specific to SMCs and mediated through direct contact and paracrine effects, respectively.
Fig. 1.
EC–SMC interactions induce different phosphorylated forms of β-catenin at different cellular compartments in ECs. (A) ECs were kept as a monoculture (EC) or cocultured with SMCs (Co) for 2, 6, 18, and 24 h, or treated with SMC-CM for 1, 6, 12, 18, and 24 h. (B) ECs were cocultured with SMCs for 24 h, and then immunostained with an anti–F-actin antibody. EC and SMC extensions within the pores were examined by confocal microscopy. ECs were cocultured with (C) SMCs on 0.02-, 0.4-, and 1.0-μm pore-size membranes for 18 h, or fibroblasts for 18 h, and (D) SMCs for 2 and 18 h. β-catenin phosphorylations and distributions were determined by Western blot. Data in A, C, D are the mean ± SEM from three to four independent experiments. Results in B are representative of three independent experiments with similar results. *P < 0.05 vs. monocultured or unstimulated control cells.
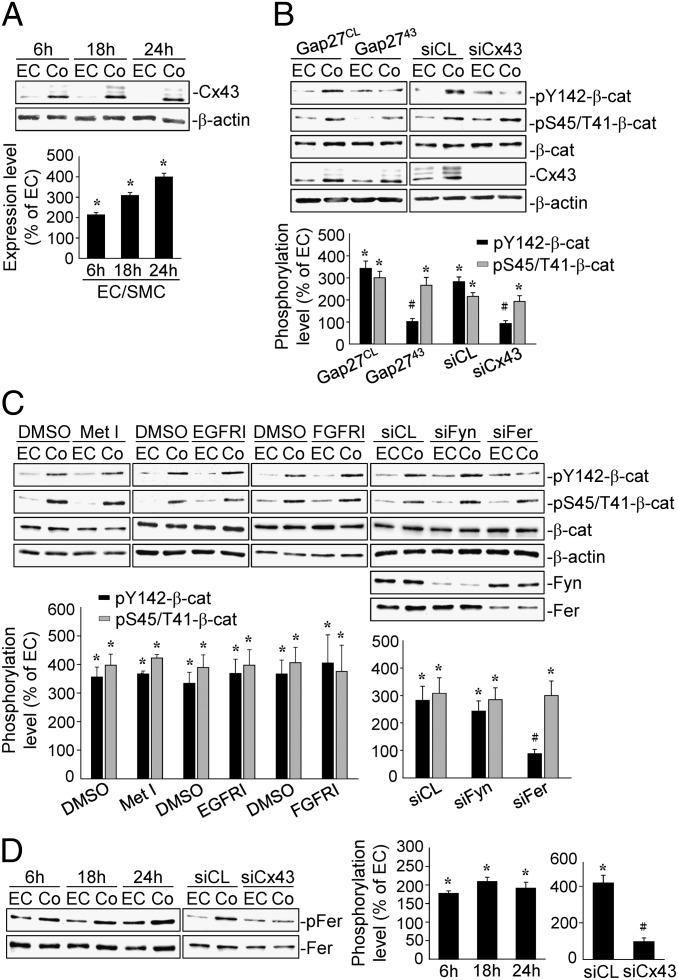
EC–SMC Contact-Induced Tyr142-Phosphorylation of β-Catenin Is Regulated by Cx43 Through Fer Phosphorylation in ECs, Independent of RTKs.
Cx43 has been shown to play critical roles in modulating EC–SMC heterocellular interactions (7). We examined the role of Cx43 in regulating EC β-catenin phosphorylation in response to EC–SMC interactions. Cx43 protein expression in ECs under the coculture increased within 6 h and persisted until 24 h (Fig. 2A). Pretreating ECs with gap2743, a Cx43 blocking peptide, or Cx43-specific siRNA before their coculture with SMCs demonstrated that inhibitions of Cx43 blocked the coculture-induced EC β-catenin phosphorylation at Tyr142, but not at Ser45/Thr41 (Fig. 2B). To determine which kinases are responsible for the coculture-induced β-catenin Tyr142-phosphorylation, ECs were pretreated with RTK inhibitors (Met, EGFR, or FGFR) and then cocultured with SMCs for 18 h. None of the RTK inhibitors blocked the β-catenin phosphorylations (Fig. 2C). Transfecting ECs with Fer-specific siRNA abolished the coculture-induced β-catenin Tyr142-phosphorylation, but not that of Ser45/Thr41 (Fig. 2C). In contrast, Fyn-specific siRNA did not have inhibitory effect. Moreover, Fer phosphorylation in ECs under EC–SMC coculture increased within 6 h and persisted until 24 h (Fig. 2D), and transfecting ECs with Cx43-specific siRNA inhibited the coculture-induced Fer phosphorylation (Fig. 2D).
Fig. 2.
The β-catenin Tyr142-phosphorylation induced by EC–SMC contact is regulated by Cx43/Fer. ECs were kept as a monoculture or cocultured with SMCs for 6, 18, and 24 h, and the Cx43 expression (A) and Fer phosphorylation (D) were determined by Western blot. ECs were pretreated with a control (Gap27CL) or Cx43 (Gap2743) blocking peptide (B) and with DMSO or RTK inhibitors (C), and transfected with control or Cx43-specific siRNA (B and D) and Fyn- or Fer-specific siRNAs (C), and then cocultured with SMCs for 18 h. β-Catenin (B and C) or Fer (D) phosphorylations were determined by Western blot. The results are the mean ± SEM from three independent experiments. *P < 0.05 vs. monocultured ECs. #P < 0.05 vs. cells pretreated with inhibitor or transfected with control siRNA.
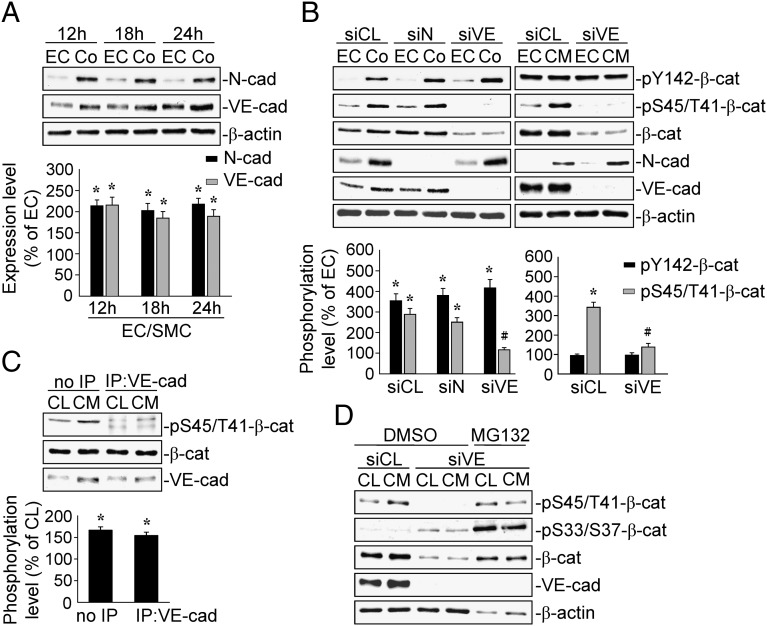
The SMC-CM–Induced EC β-Catenin Ser45/Thr41-Phosphorylation Is Mediated by VE-Cadherin Association, Which Prevents the Ubiquitin-Dependent Degradation of Ser45/Thr41-Phosphorylated β-Catenin.
We examined whether N- or VE-cadherin mediate the EC β-catenin phosphorylations induced by EC–SMC coculture. The expressions of N- and VE-cadherin proteins increased within 12 h and persisted until 24 h after EC–SMC coculture (Fig. 3A). Transfecting ECs with specific VE-cadherin–siRNA inhibited the coculture- and SMC-CM–induced EC phosphorylations of β-catenin at Ser45/Thr41, but not at Tyr142 (Fig. 3B). The knockdown of VE-cadherin with specific siRNA also blocked the basal levels of β-catenin Ser45/Thr41-phosphorylation in ECs. In contrast, N-cadherin–specific siRNA had no inhibitory effect. Application of SMC-CM to ECs for 18 h induced Ser45/Thr41-phosphorylation of β-catenin associated with VE-cadherin (Fig. 3C). To determine whether VE-cadherin plays a role in protecting β-catenin from degradation, we incubated ECs with VE-cadherin–specific siRNA, pretreated them with MG132—which is a ubiquitin-dependent proteasome inhibitor—and then treated them with SMC-CM for 18 h. The VE-cadherin–specific siRNA transfection decreased β-catenin Ser45/Thr41-phosphorylation and protein expression, and increased β-catenin Ser33/37-phosphorylation, which is a well-known self-degradation signal, in both the SMC-CM–treated and –untreated ECs (Fig. 3D). Pretreatment with MG132 inhibited the effects of VE-cadherin knockdown on β-catenin Ser45/Thr41-phosphorylation and protein expression, but further increased the β-catenin Ser33/37-phosphorylation in the VE-cadherin–specific siRNA-transfected ECs to levels higher than those pretreated with DMSO.
Fig. 3.
The EC β-catenin Ser45/Thr41-phosphorylation induced by SMC-CM is mediated by VE-cadherin association. (A) ECs were kept as a monoculture or cocultured with SMCs for 12, 18, and 24 h, and the expressions of N- and VE-cadherin were determined by Western blot. (B) ECs were transfected with control, N-, or VE-cadherin–specific siRNAs. (C) The association between VE-cadherin and phospho-Ser45/Thr41–β-catenin was determined by immunoprecipitation and Western blot. (D) ECs were transfected with control or VE-cadherin-specific siRNA and pretreated with MG132, and then cocultured with SMCs or treated with SMC-CM for 18 h. Data in A–C are the mean ± SEM from three independent experiments. Results in D are representative of three independent experiments with similar results. *P < 0.05 vs. monocultured or unstimulated control cells; #P < 0.05 vs. cells transfected with control siRNA.
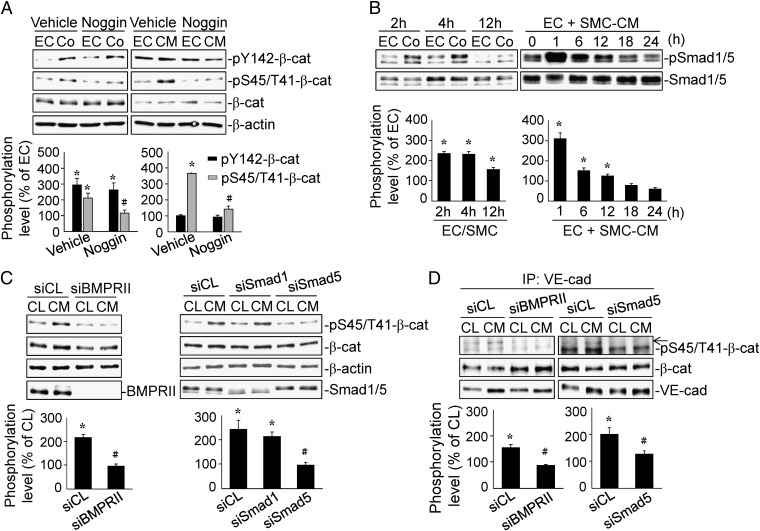
SMC-CM–Induced Ser45/Thr41-Phosphorylation of β-Catenin Is Mediated by SMC-Released BMPs Through BMPRII and Smad5 in ECs.
BMPs have been found to integrate β-catenin signaling to modulate EC and SMC functions (4). We determined whether SMCs release BMPs to modulate EC β-catenin phosphorylations under EC–SMC interactions by treating the cells with Noggin, a specific BMPs antagonist. Noggin treatment inhibited coculture- and SMC-CM–induced β-catenin phosphorylation at Ser45/Thr41, but not Tyr142 (Fig. 4A). Moreover, both coculture and SMC-CM–treatment induced rapid increases (within 2 h and 1 h, respectively) in Smad1/5 phosphorylation, which declined after 12 h (Fig. 4B). Transfecting ECs with specific siRNA of BMPRII and Smad5, but not Smad1, decreased the SMC-CM–induced β-catenin Ser45/Thr41-phosphorylation (Fig. 4C). Coimmunoprecipitation assay with specific VE-cadherin antibody further demonstrated that BMPRII and Smad5 gene knockdown in ECs markedly inhibited the SMC-CM–induced VE-cadherin–associated β-catenin phosphorylation at Ser45/Thr41 (Fig. 4D).
Fig. 4.
The Ser45/Thr41-phosphorylation of β-catenin in ECs induced by SMC-CM is mediated by SMC-released BMPs through BMPRII/Smad5. (A) ECs were pretreated with vehicle or Noggin, and then treated with SMC-CM for 18 h. (B) ECs were kept as a monoculture or cocultured with SMCs for 2, 4, and 12 h or treated with SMC-CM for 1, 6, 12, 18, and 24 h. (C) ECs were transfected with control, BMPRII-, Smad1-, or Smad5-specific siRNA, and then treated with SMC-CM for 18 h. β-catenin (A and C) and Smad1/5 (B) phosphorylations were determined by Western blot. The VE-cadherin–associated β-catenin Ser45/Thr41 phosphorylation was determined by immunoprecipitation and Western blot (D). The results are the mean ± SEM from three independent experiments. *P < 0.05 vs. monocultured or unstimulated control cells; #P < 0.05 vs. cells pretreated with vehicle or transfected with control siRNA.
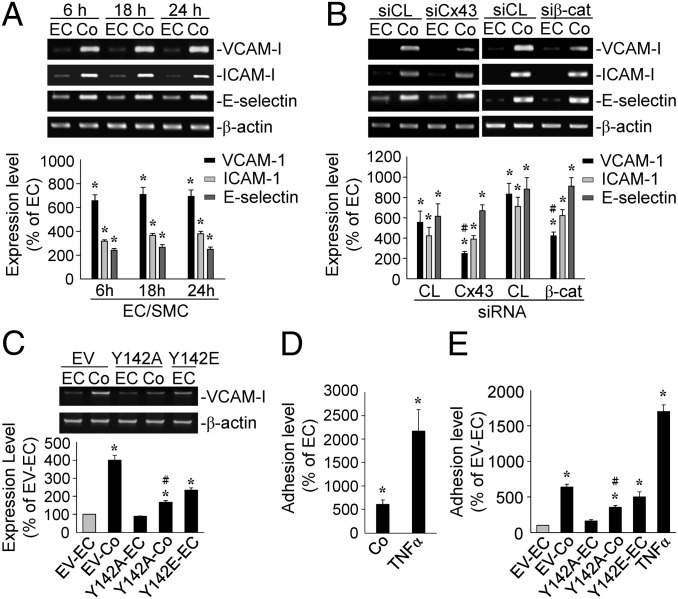
Tyr142-Phosphorylation of β-Catenin in ECs Induced by Direct Contact with SMCs Is Critical for SMC-Induction of EC VCAM-1 and Monocyte Adhesion.
EC–SMC coculture induced expressions of VCAM-1, intercellular adhesion molecule-1 (ICAM-1), and E-selectin genes within 6 h and persisted until 24 h (Fig. 5A). Transfecting ECs with Cx43- or β-catenin–specific siRNA inhibited the coculture-induced VCAM-1 expression (Fig. 5B). Transfecting BAECs with a dominant-negative mutant of β-catenin-Tyr142 (Y142A) (compared with an empty vector) reduced the VCAM-1 gene expression induced by coculture with BASMCs (Fig. 5C). In contrast, transfecting BAECs with a dominant-positive mutant of β-catenin–Tyr142 (Y142E) increased VCAM-1 expression in the monocultured BAECs. The adhesion of THP-1 monocytic cells to ECs cocultured with SMCs increased ∼6.5-fold in comparison with the monocultured ECs (Fig. 5D). Transfecting BAECs with a β-catenin–Tyr142 mutant (Y142A; compared with empty vector) inhibited THP-1 adhesion to the cocultured BAECs (Fig. 5E). Transfecting BAECs with a β-catenin–Tyr142 mutant (Y142E) increased THP-1 adhesion to the monocultured BAECs.
Fig. 5.
Tyr142-phosphorylation of β-catenin in ECs induced by direct contact with SMCs is critical for SMC-induction of EC VCAM-1 and monocytic adhesion. (A) ECs were kept as a monoculture or cocultured with SMCs for 6, 18, and 24 h. (B) ECs were transfected with control, Cx43-, or β-catenin–specific siRNA. (C and E) ECs were transfected with a control empty vector or β-catenin mutants (Y142A or Y142E) and then kept as a monoculture or cocultured with SMCs for 18 h. (D) ECs were kept as a monoculture, cocultured with SMCs for 18 h, or activated with TNF-α for 4 h. The VCAM-1, ICAM-1, and E-selectin gene expressions were determined by RT-PCR (A–C) and the monocytic adhesion in each sample were counted (D and E). The results are the mean ± SEM from three independent experiments. *P < 0.05 vs. monocultured ECs; #P < 0.05 vs. cells transfected with control siRNA or empty vector.
Ser45/Thr41-Phosphorylation of β-Catenin in ECs Induced by Paracrine Interaction with SMCs Is Critical for Modulating EC Permeability.
As indicated in SI Results, SMC-CM increased the EC permeability to FITC-dextran, and this effect was attenuated by transfecting BAECs with BMPRII- and Smad5-specific siRNAs or a dominant-negative mutant of β-catenin–Ser45 (Fig. S3).
Discussion
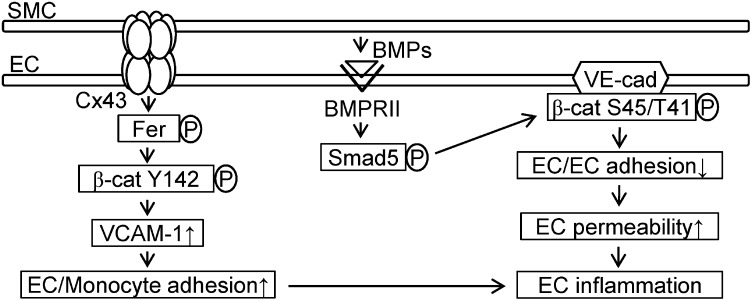
Our present study has made a unique finding that two distinct modes of phosphorylation on β-catenin (i.e., Tyr142 and Ser45/Thr41) can be differentially triggered by different types of EC–SMC interaction, with the consequent modulations in EC functions (summarized in Fig. 6). These conclusions were based on the following results: (i) EC–SMC heterocellular interactions induce the EC phosphorylation of both β-catenin–Tyr142 at the cytoplasm/nuclei and β-catenin–Ser45/Thr41 at the membrane through direct contact and paracrine effect, respectively. (ii) EC–SMC direct contact-induced EC β-catenin Tyr142-phosphorylation is mediated by membrane Cx43 through cytosolic Fer kinase in ECs. This phosphorylated form of β-catenin under EC–SMC direct contact has the important role of increasing VCAM-1 gene expression. (iii) The paracrine effect of SMC-released BMPs on ECs results in BMPRII/Smad5 activation to induce VE-cadherin–associated β-catenin phosphorylation at Ser45/Thr41. (iv) Both modes of β-catenin phosphorylation in ECs induced by SMCs result in EC inflammation, but with different consequences: Tyr142-phosphorylation increases monocytic adhesion to ECs, whereas Ser45/Thr41-phosphorylation modulates EC–EC junctional integrity to increase EC permeability. Our findings provide new insights that different phosphorylated forms of β-catenin, which are triggered by different EC–SMC interactions through direct contact and paracrine effect, have distinct roles in mediating EC inflammation.
Fig. 6.
Schematic diagram showing distinct mechanisms of EC β-catenin phosphorylation and the subsequent modulation in EC inflammation through direct contact and paracrine interactions with SMCs.
There are three types of junctional structures (gap, adherens, and tight junctions) involved in the modulation of cell–cell direct interaction. Gap junction is the main communication structure for EC–SMC direct contact through myoendothelial junction (7). Cx is a major gatekeeper of gap junction and responsible for the intercellular delivery of molecules and ions (7, 9, 10). Our study indicates that β-catenin Tyr142-phosphorylation induced by EC–SMC direct contact is mediated by Cx43 through Fer kinase in ECs, suggesting that Cx43 might function as a signaling molecule to initiate such β-catenin phosphorylation. In addition to the Cx/gap junction, the N-cadherin/adherens junction has also been shown to modulate EC–SMC direct interaction (7, 8). However, we did not find SMC Cx43 and N-cadherin to play a role in modulating EC β-catenin Tyr142-phosphorylation induced by EC–SMC direct contact, inasmuch as transfection of SMCs with Cx43- and N-cadherin-specific siRNAs did not affect the EC–SMC contact-induced β-catenin Tyr142-phosphorylation in ECs (Fig. S4). Thus, our study has not yet identified the communicators from SMCs that can interact with Cx43 in ECs to induce their β-catenin Tyr142-phosphorylation. It has been shown that a gap junction can be formed through the homo- and hetero-modes. Whether other Cx proteins, such as Cx40 and Cx45, are the responsible molecules in the SMC side to interact with EC Cx43 (7) remains to be determined. In addition, accumulating evidence indicates that the tugging force generated in the process of cell–cell contact may modulate the junction structure (11). Therefore, the junctional forces may also be another candidate in modulating EC β-catenin phosphorylation and warrants further exploration.
It has been demonstrated that β-catenin Tyr-phosphorylation may disrupt its junctional role and promote β-catenin translocation to the cytoplasm/nuclei to increase its transcription cofactor activity (1, 3). However, our present studies found that the increased Tyr142-phosphorylation induced by EC–SMC direct contact occurred only at the cytosolic and nuclear β-catenin, but not at the membrane, and that there was no translocation of β-catenin. In this connection, it is to be noted that Wnt-induced changes in β-catenin at the cytosolic compartment have been found not to be the form of β-catenin associated with cadherin at the membrane in tumor cells (12). Hence, our results would suggest that the β-catenin resulting from EC–SMC direct contact stimulation may be distinct from that found at the membrane. Our functional investigation of Tyr142-phosphorylation on β-catenin in direct contact model shows that such phosphorylation plays a critical role in increasing VCAM-1 expression and hence monocytic adhesion to ECs. Previous studies by others and us have demonstrated that NF-κB is the major transcription factor regulating the EC expression of adhesion molecules, including VCAM-1 (6). The synergistic effects of β-catenin and NF-κB have been found in development and diseases (13). Hence, it is possible that β-catenin serves as a cofactor to enhance NF-κB transcriptional activity under EC–SMC direct contact.
In addition to Tyr142 phosphorylation, Ser45 phosphorylation on β-catenin also plays an important role in modulating its activity. Our study has shown that the mechanism of Ser45 phosphorylation of β-catenin is different from that of Tyr142 phosphorylation and is triggered by a different EC–SMC interaction mode (i.e., paracrine). The results indicate that the β-catenin Ser45/Thr41-phosphorylation induced by EC–SMC paracrine interaction modulates the junctional role of β-catenin at EC membrane, but does not lead to its degradation. Recent studies have also indicated that N-terminal Ser45 phosphorylation of β-catenin does not always induce the autodegradation cascade (2). Our study on the molecular mechanism of EC–SMC interaction shows that membrane VE-cadherin may serve as an anchor protein to protect the Ser45/Thr41-phosphorylated form of β-catenin from degradation, because (i) this phosphorylation only occurs on VE-cadherin-associated β-catenin and (ii) gene knockdown of VE-cadherin decreases β-catenin protein level. Our results suggest that the role of the Ser45/Thr41-phosphorylated form of β-catenin is to modulate the VE-cadherin–controlled junction structure, and hence cause an attenuation of EC–EC junction to increase EC permeability. These studies advance the notions that β-catenin Ser45-phosphorylation is a complicated regulatory mechanism on β-catenin activity and, hence, the functional fate of the cell and that the N-terminal Ser45-phosphorylated form of β-catenin may serve as an important modulator for EC–EC interactions.
BMPs/BMPRII/Smad1 and -5 signaling plays important roles in the pathogenesis of several diseases (14). Recent studies indicate that BMPs, which can be secreted by both vascular ECs and SMCs, are highly expressed in atherosclerotic lesions and play important roles in regulating calcification and inflammation in the vascular well (15). Inhibition of BMPs protects against atherosclerosis and vascular calcification (15, 16). In contrast to the proinflammatory role of BMPs, a recent study by Kim et al. (17) demonstrated that BMPRII plays a constitutively anti-inflammatory and antiatherogenic role in ECs. Loss of BMPRII expression causes endothelial inflammation and atherosclerosis (17). In the present study, we did not attempt to define the in vivo role of BMPRII in endothelial and vascular functions. Our data showed that SMCs may release BMPs to induce Ser45/Thr41-phosphorylation of VE-cadherin–associated β-catenin in ECs through BMPRII and Smad5, thus affecting EC permeability. It remains to be determined whether the sensing of BMP signaling by BMPRII in the plaques plays a dual role in modulating EC inflammation and permeability through differential mechanisms in vivo, with the net result of an atheroprotective function.
Our in vitro findings have the following physiological significance. During atherosclerotic lesion development, SMCs change from their physiological contractile phenotype to the pathophysiologic synthetic phenotype and migrate into the intima, where they release proinflammatory factors and interact with ECs to regulate their gene expression and function. Because the SMCs used in our study are in a synthetic phenotype (6), our results on the increased permeability of ECs induced by their paracrine interactions with synthetic SMCs through the modulation in EC β-catenin Ser45/Thr41-phosphorylation suggest that EC β-catenin Ser45/Thr41-phosphorylation may contribute to the pathogenesis of atherosclerosis. In addition, given the increasing evidence that myoendothelial junctions through which ECs and SMCs make direct contact play critical roles in regulating vascular biology and nitric oxide function (7, 18), our current findings provide unique mechanisms by which different modes of EC–SMC heterocellular interactions regulate vascular inflammation and barrier function through differential phosphorylations of EC β-catenin at Tyr142 and Ser45/Thr41.
In conclusion, the present study has demonstrated that EC–SMC interactions involve direct contact and paracrine effects, which lead to distinct phosphorylations of β-catenin at diverse cellular locations and different functional consequences. EC–SMC direct contact causes β-catenin phosphorylation at Tyr142 in cytoplasm/nuclei to enhance VCAM-1 expression and EC-monocytic adhesion. In contrast, the paracrine effect causes β-catenin phosphorylation at Ser45/Thr41 in the membrane to affect EC–EC junction integrity and increase permeability. These results provide new insights into the understanding of the complexities of β-catenin phosphorylation signaling underlying the EC–SMC heterocellular interactions. The notion that the phosphorylated forms of β-catenin play differential roles in modulating vascular pathophysiology suggests that β-catenin may be a promising molecular target for therapeutic intervention against vascular disorders associated with EC dysfunction.
Materials and Methods
Cell Culture.
Human umbilical vein endothelial cells were isolated from fresh human umbilical cords by collagenase perfusion and grown in Petri dishes in Medium 199 supplemented with 20% (vol/vol) FBS for 3 d. Human umbilical artery SMCs and BAECs/BASMCs were obtained commercially (Clonetics) and maintained in F12K and maintained in DMEM, respectively, supplemented with 10% (vol/vol) FBS. Human skin fibroblasts were purchased from American Type Culture Collection and maintained in MEM supplemented with 10% (vol/vol) FBS and l-glutamine.
Coculture Modules.
Cocultures were established by plating cells on opposite sides of porous polyethylene terephthalate membrane (Falcon Cell Culture Inserts; Becton Dickinson), as previously described (6). Briefly, ECs were seeded onto the outer side of a membrane precoated with fibronectin. After allowing 2 h for adherence, the membrane was placed with the EC side down into a six-well plate containing the culture medium, and the opposite side of the membrane was seeded with SMCs to form the EC–SMC coculture. Controls included ECs as above but no SMCs on the inner side of the membrane.
The detailed materials and methods are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by NSC-100-2325-B-400-011/100-2321-B-400-001 (to J.-J.C.); HL-106579/HL-108735 (to S.C.); and NSC-101-2911-I-009-101 to University System of Taiwan–University of California at San Diego International Research-Intensive Center of Excellence in Advanced Bioengineering (to S.C. and J.-J.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323761111/-/DCSupplemental.
References
- 1.Daugherty RL, Gottardi CJ. Phospho-regulation of β-catenin adhesion and signaling functions. Physiology (Bethesda) 2007;22:303–309. doi: 10.1152/physiol.00020.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faux MC, Coates JL, Kershaw NJ, Layton MJ, Burgess AW. Independent interactions of phosphorylated β-catenin with E-cadherin at cell-cell contacts and APC at cell protrusions. PLoS ONE. 2010;5(11):e14127. doi: 10.1371/journal.pone.0014127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piedra J, et al. p120 Catenin-associated Fer and Fyn tyrosine kinases regulate β-catenin Tyr-142 phosphorylation and β-catenin-α-catenin Interaction. Mol Cell Biol. 2003;23(7):2287–2297. doi: 10.1128/MCB.23.7.2287-2297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Jesus Perez VA, et al. Bone morphogenetic protein 2 induces pulmonary angiogenesis via Wnt-beta-catenin and Wnt-RhoA-Rac1 pathways. J Cell Biol. 2009;184(1):83–99. doi: 10.1083/jcb.200806049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segal SS, Duling BR. Conduction of vasomotor responses in arterioles: A role for cell-to-cell coupling? Am J Physiol. 1989;256(3 Pt 2):H838–H845. doi: 10.1152/ajpheart.1989.256.3.H838. [DOI] [PubMed] [Google Scholar]
- 6.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91(1):327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heberlein KR, Straub AC, Isakson BE. The myoendothelial junction: Breaking through the matrix? Microcirculation. 2009;16(4):307–322. doi: 10.1080/10739680902744404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navarro P, Ruco L, Dejana E. Differential localization of VE- and N-cadherins in human endothelial cells: VE-cadherin competes with N-cadherin for junctional localization. J Cell Biol. 1998;140(6):1475–1484. doi: 10.1083/jcb.140.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ale-Agha N, et al. HuR regulates gap junctional intercellular communication by controlling beta-catenin levels and adherens junction integrity. Hepatology. 2009;50(5):1567–1576. doi: 10.1002/hep.23146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vinken M, et al. Connexins: Sensors and regulators of cell cycling. Biochim Biophys Acta. 2011;1815(1):13–25. doi: 10.1016/j.bbcan.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Liu Z, et al. Mechanical tugging force regulates the size of cell-cell junctions. Proc Natl Acad Sci USA. 2010;107(22):9944–9949. doi: 10.1073/pnas.0914547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottardi CJ, Gumbiner BM. Distinct molecular forms of β-catenin are targeted to adhesive or transcriptional complexes. J Cell Biol. 2004;167(2):339–349. doi: 10.1083/jcb.200402153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho HH, Joo HJ, Song JS, Bae YC, Jung JS. Crossregulation of β-catenin/Tcf pathway by NF-kappaB is mediated by lzts2 in human adipose tissue-derived mesenchymal stem cells. Biochim Biophys Acta. 2008;1783(3):419–428. doi: 10.1016/j.bbamcr.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Chang SF, et al. Tumor cell cycle arrest induced by shear stress: Roles of integrins and Smad. Proc Natl Acad Sci USA. 2008;105(10):3927–3932. doi: 10.1073/pnas.0712353105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao Y, et al. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circ Res. 2010;107(4):485–494. doi: 10.1161/CIRCRESAHA.110.219071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derwall M, et al. Inhibition of bone morphogenetic protein signaling reduces vascular calcification and atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(3):613–622. doi: 10.1161/ATVBAHA.111.242594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim CW, et al. Anti-inflammatory and antiatherogenic role of BMP receptor II in endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33(6):1350–1359. doi: 10.1161/ATVBAHA.112.300287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Straub AC, et al. Endothelial cell expression of haemoglobin α regulates nitric oxide signalling. Nature. 2012;491(7424):473–477. doi: 10.1038/nature11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.