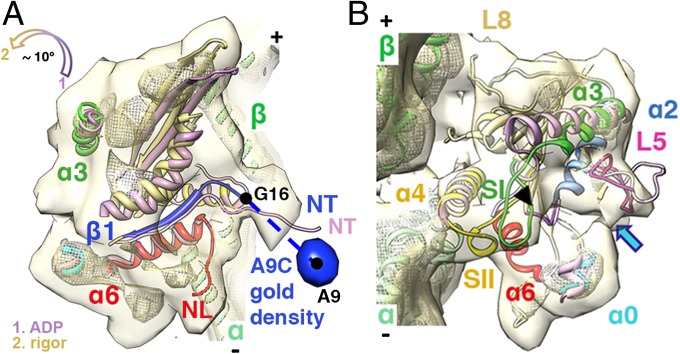
Fig. 3.
Conformational changes associated with ADP release. (A and B) K5 MD pseudo-atomic models of the ADP (light purple) and rigor states are superimposed on helices α4 and docked into the rigor reconstruction (15); surface: 0.94σ contour; mesh: 3σ contour. (A) The position of the gold density (blue surface, 1.4σ contour) attached to the N terminus via A9C at ∼23 Å or approximately seven amino acids away from the first residue in our rigor model (G16) confirms our previous assignment of the N terminus in the rigor reconstruction (15). The color-coded arrow indicates the direction of the MD tilt around the helix α4 and towards the MT lattice upon ADP release. (B) Upon ADP release, L5 moves towards the empty nucleotide-binding pocket, based on superimposition of helices α2. The black arrowhead and the blue arrow indicate a connection between L5 and switch I (SI) and between L5 and helix α0, respectively. Helix α3/switch I, helix α4, and helix α0 of the ADP model also are shown for comparison.