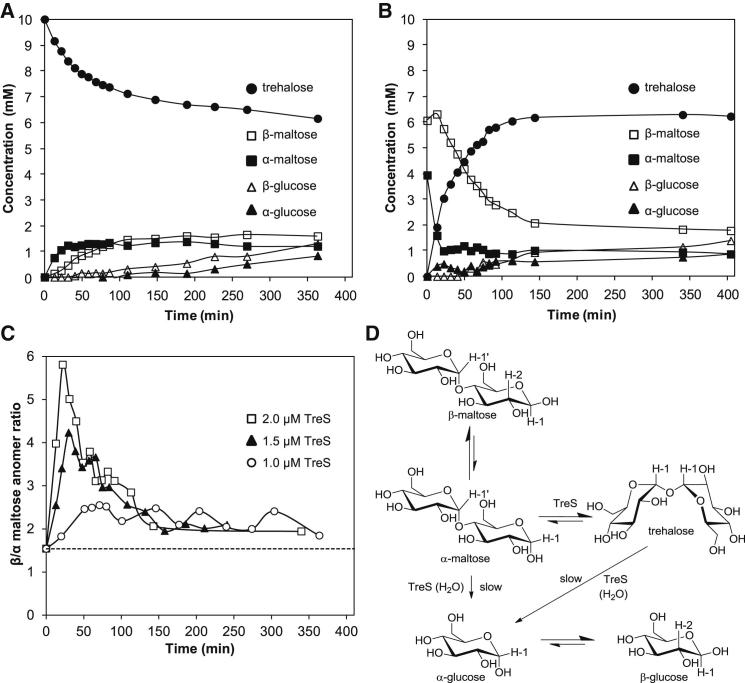
Figure 3.
Mycobacterium Tuberculosis TreS Interconverts the α Anomer of Maltose
(A) The conversion of trehalose (10 mM) into maltose by TreS (2 μM) according to 1H-NMR spectroscopy (see Figures S2A–S2C for representative spectra and nonenzymic mutarotation controls). Each component was quantified by signal integration using citrate as an internal standard. Reaction mixtures contained 10% D2O to assist spectrum acquisition without significant risk of introducing solvent kinetic and/or equilibrium isotope effects. This necessitated solvent suppression and the introduction of experimentally determined correction factors for the cosuppression of resonances that were close to the solvent resonance.
(B) The conversion of pre-equilibrated α/β-maltose (10 mM) into trehalose by TreS (2 μM) according to 1H-NMR spectroscopy. Note that enzyme was added immediately after the t = 0 data were acquired, resulting in a small and reproducible change in the apparent concentration of starting materials at the second recorded time point. See Figure S2D for the fit of the β-maltose curve (Hoops et al., 2006). The times taken to produce 2 mM trehalose and consume 50% of the α/β-maltose were 14 and 55 min, respectively.
(C) Time courses of the ratios between the β and α anomers of maltose with different TreS concentrations during the conversion of pre-equilibrated α/β-maltose. The broken line indicates the equilibrium between the two anomers in these conditions.
(D) Proposed reaction scheme to account for TreS-catalyzed reactions. Protons used to quantify components of the reaction mixtures by 1H-NMR spectroscopy (Figure S2A) are indicated.