Figure 2.
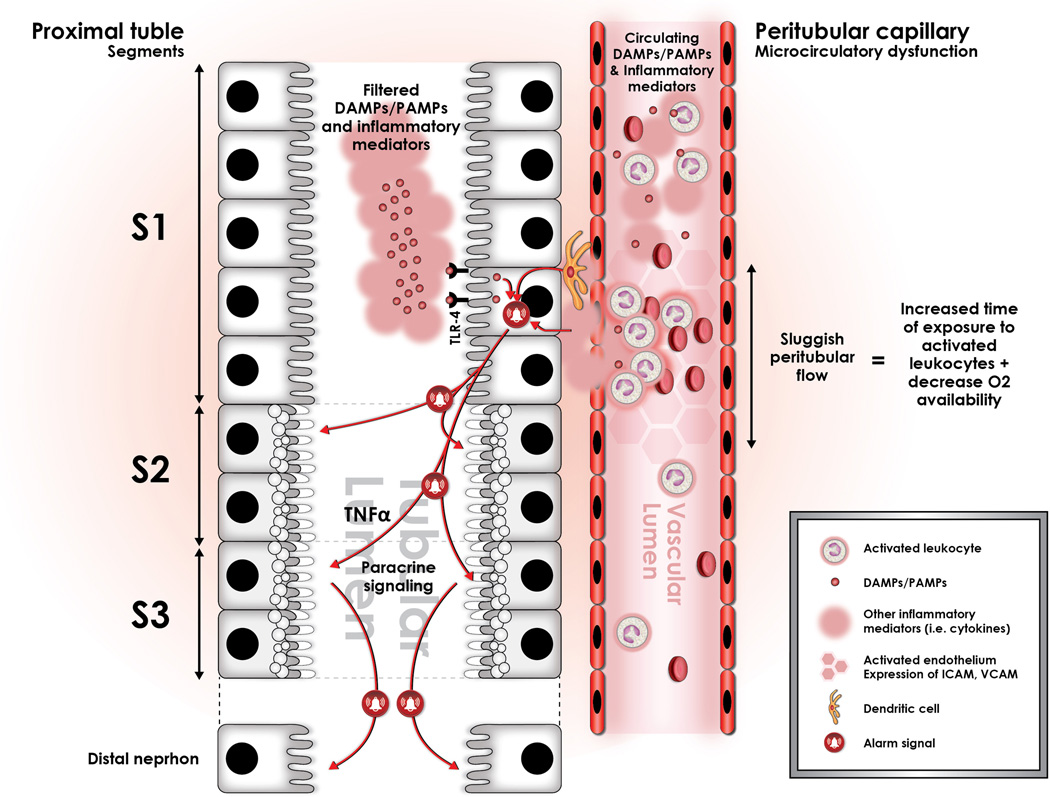
Sepsis induces profound alterations in microcirculatory flow in the entire organism, and the kidney is not the exception. This alteration is characterized by a significant increment in the heterogeneity of flow, as well as an increase in the proportion of capillaries with sluggish or stop flow (represented in the figure by darker hexagons in the peritubular capillary). We have conceptualized that these areas of sluggish peritubular flow increase the transit time of activated, cytokine spilling leukocytes, and that this may set the stage for an amplification of the “danger signal” in such areas. These areas of sluggish flow have been shown to co-localize with expression of oxidative stress in the tubular epithelial cells, suggesting causation. In addition, immuno-histologic studies have shown that oxidative stress is localized to the apex of the tubular epithelial cell, and that it is associated to the formation of apical vacuoles as represented hereby in the figure. Importantly, this may explain the mechanism by which apical vacuoles are formed during sepsis-induced AKI and also the histologic phenotype. In addition, filtered LPS is recognized by S1 tubular epithelial cells through TLR-4 and is internalized via endocytosis. This event has been shown to trigger an oxidative outburst, not in the S1 segment cells, but rather in the S2 segment cells. This seems to be associated with the expression in S1, but not in S2 epithelial cells of Heme-oxigenase 1 (HO-1) and Sirt1, both highly protective against oxidative damage.22,80 In addition, expression of TNF receptors in the S2 segment tubular cells has led to the hypothesis that S1 cells may signal distal segments in a paracrine fashion through secretion of TNF-alpha. Finally, there is also data suggesting that this paracrine signal may also include mediators of cell cycle arrest, namely TIMP-2 and IGFBP-7.