Figure 1. Effect of Mouse β-defensin 2 (Mbd2) on the humoral immune responses induced by HAd-HA-NP.
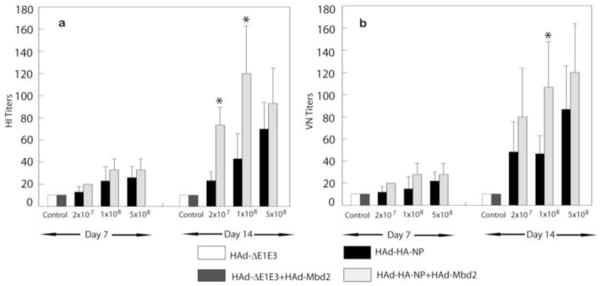
Groups of mice (N=11) were intramuscularly vaccinated with 2 × 107, 1 × 108, or 5 × 108 plaque forming units (pfu) of HAd-HA-NP with or without 1 × 107 pfu of HAd-Mbd2. Animals that similarly received 1 × 108 pfu of the HAd-ΔE1E3 or 1 × 108 pfu of HAd-ΔE1E3 + 1 × 107 pfu of HAd-Mbd2 served as controls. At Days 7 or 14 post-immunization, serum samples were obtained and analyzed for hemagglutination inhibition (HI) (1a) and virus neutralizing (VN) antibody titers (1b) by HI and microneutralization assays, respectively. The data represent mean antibody titers ± standard deviation (SD) from six animals per group. *, P≤0.05 compared to mice vaccinated with a similar dose of HAd-HA-NP.
