Abstract
Background aims
The distinction between hematopoietic stem cells (HSC) and endothelial progenitor cells (EPC) is poorly defined. Co-expression of CD34 antigen with vascular endothelial growth factor (VEGF) receptor (VEGFR2) is currently used to define EPC (1).
Methods
We evaluated the phenotypic and genomic characteristics of peripheral blood-derived CD34+ cells in 22 granulocyte–colony-stimulating factor (G-CSF)-mobilized patients with severe coronary artery disease and assessed the influence of cell selection and storage on CD34+ cell characteristics.
Results
The median CD34+ cell contents in the products before and after enrichment with the Isolex 300i Magnetic Cell Selection System were 0.2% and 82.5%, respectively. Cell-cycle analysis showed that 80% of CD34+ cells were in G0 stage; 70% of the isolated CD34+ cells co-expressed CD133, a marker for more immature progenitors. However, less than 5% of the isolated CD34+ cells co-expressed the notch receptor Jagged-1 (CD339) and only 2% of the isolated CD34+ population were positive for VEGFR2 (CD309). Molecular assessment of the isolated CD34+ cells demonstrated extremely low expression of VEGFR2 and endothelial nitric oxide synthase (eNOS) and high expression of VEGF-A. Overnight storage at 4°C did not significantly affect CD34+ cell counts and viability. Storage in liquid nitrogen for 7 weeks did not affect the percentage of CD34+ cells but was associated with a 26% drop in cell viability.
Conclusions
We have demonstrated that the majority of isolated CD34+ cells consist of immature and quiescent cells that lack prototypic markers of EPC. High VEGF-A gene expression might be one of the mechanisms for CD34+ cell-induced angiogenesis.
Keywords: coronary artery disease, endothelial progenitor cells, stem cell mobilization, transplant
Introduction
The capacity of hematopoietic stem cells (HSC) to transdifferentiate to form non-hematopoietic tissue has been well studied and reported by us and others (2,3). Unlike these non-hematopoietic tissues, endothelial cells are thought to share a common precursor with HSC during fetal development, called the hemangioblast. Although definitive evidence for the origin of the hemangioblast is lacking, indirect evidence for such cells in mice and chicks exists. Eichmann et al. (4) have studied mesoderm-derived clonal cultures and shown that deletion of vascular endothelial growth factor receptor (VEGFR2) results in elimination of both endothelial and hematopoietic cells in homozygous mice. Similarly, Choi et al. (5) have shown that embryoid bodies derived from embryonic stem cells contain a unique precursor population that, in response to soluble vascular endothelial growth factor (VEGF), gives rise to blast colonies that generate both hematopoietic and endothelial precursor cells. It is unclear at what stage in hemangioblast development HSC and endothelial progenitor cells (EPC) emerge.
EPCs are a population of cells found in the peripheral circulation (6,7). Isolation of circulating EPC for angiogenesis was first described by Asahara et al. in 1997 (6). This prompted a series of experimental studies demonstrating that EPC contribute to angiogenesis and vasculogenesis, thereby leading to attenuation of myocardial remodeling and improvement of left ventricular function (7,8). However, the numbers of EPC in the peripheral circulation of healthy adults are scant (9) and further reduced in disease states (10) such as coronary atherosclerosis (11), cardiac transplant vasculopathy (12), instent restenosis (13) and diabetes (14). This is in contrast to other studies that have demonstrated a rapid increase of EPC in the peripheral circulation after acute vascular injury such as limb ischemia (15), myocardial infarction (16) and coronary artery bypass grafting (17). Even patients with unstable angina and no evidence of cardiac necrosis have been shown to exhibit increased circulating EPC (18), suggesting that systemic inflammation could play a role in the peripheral mobilization of EPC. Moreover, mobilization of EPC in vivo has been stimulated after treatment with 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase inhibitors (19,20) and application of growth factors such as granulocyte–colony-stimulating factor (G-CSF) (15) and VEGF (21).
Although many studies on EPC have been published, a critical marker for identification of EPC has not been established. VEGF and VEGFR2 have been used as possible markers. The VEGF gene is located on chromosome 6 and exists in multiple isoforms of variable axon content (22). VEGF-A, one of the isoforms, performs its various biologic functions through its interactions with two signaling tyrosine kinases, VEGFR1 (Flt-1) and VEGFR2 (CD309, KDR, Flk-1) (23). VEGF-A expression is induced by hypoxia, various cytokines such as G-CSF (24), sex hormones and chemokines (25). Previous studies suggest that VEGFR1 is important in vascular remodeling (26) and VEGFR2 is a critical receptor for transmitting cellular signals for induction of proliferation and differentiation in endothelial cells (27). Hence these endothelial-related markers may have potential as critical markers of EPC.
In this study, we used CD133, VEGFR2 and CD339 (notch/Jagged-1) to analyze isolated CD34+ cells phenotypically. Co-expression of CD133 in CD34+ cells has been associated with early HSC (28) and long-term engraftment (29). The CD133+ cell population consists of stem/progenitor cells not only with hematopoietic potential but also with the capacity to differentiate into EPC. (30) Also, the population, VEGFR2 co-expressed with CD34 antigen, is considered to be the prototypical EPC phenotype (1). However, CD339, known as Jagged-1, is a notch receptor the signaling pathway of which is critical in HSC differentiation and proliferation (31). Presumably, CD339 expression will be high in committed, differentiating and proliferating CD34+ cells, and low or absent in quiescent, primitive CD34+ cells. Therefore, we thought this marker would be useful in distinguishing primitive from committed HSC.
EPC characterization was assessed by evaluating gene expression of endothelial nitric oxide synthase (eNOS), VEGFR2 and VEGF-A, as these have been associated with angiogenesis and endothelial function (32–34). Cell-cycle analysis was also carried out. It is generally believed that stem cells are usually quiescent (G0) and only briefly enter the cell cycle when there is demand for tissue repair or replenishment (35). In addition, quiescent CD34+ cells are considered to be primitive HSC and have been correlated with long-term hematopoietic reconstitution (36).
G-CSF is the principal growth factor that is used clinically to mobilize hematopietic stem and progenitor cells (HSPC) from marrow to peripheral blood for collection by leukapheresis. Engagement of GCSF to its target cells indirectly decreases stromal derived factor-1a (SDF1a) production in the marrow and this allows HSC to migrate to the peripheral blood (37). G-CSF-induced protease enzymes such as neutrophil elastase and MMP-9 are known to induce HSC to migrate out of their bone marrow (BM) niche to peripheral blood (38). In addition, G-CSF improves CD34+ cell mobilization and angiogenesis by stimulating VEGF secretion from neutrophils (39,40). In this study we characterized human autologous G-CSF-mobilized peripheral blood CD34+ cells that were used to induce angiogenesis and tissue repair in patients with severe coronary artery disease with persistent but stable angina.
Methods
Patient characteristics
Blood samples were collected from 22 autologous hematopoietic progenitor cell (HPC) donors. The median patient age was 62 years (range 45–76), and 16 of the 22 patients were males. The median patient body weight was 101.4 kg. All patients were diagnosed with severe coronary artery disease that was unresponsive to conventional treatment (Table I). All study subjects underwent one HPC collection. Mayo Clinic (Jacksonville, FL, USA) Institutional Review Board (IRB) (IRB# 0260) approvals were obtained before the study was conducted. All patients signed a specific informed consent giving permission to use the excess blood remaining after apheresis and not required for study treatment by the Mayo Clinic lead investigator (Abba Zubair) for research purposes. These subjects were participants in the ACT34-CMI phase II trial (Table II). ACT34- CMI is a double-blind, prospective, randomized, placebo-controlled study that aims to determine the tolerability, efficacy, safety and dose range of intra-myocardial injections of G-CSF-mobilized auto-CD34+ cells for reduction of angina episodes in subjects with treatment-refractory chronic myocardial ischemia.
Table I.
Donor and graft characteristics.
| Demographics | Numbers (range) |
|---|---|
| Sample size | 22 |
| Donor age | 62 (45–76) |
| Weight (kg) | 101.4 (57.7–129.5) |
| Males | 16 |
| Females | 6 |
| Co-morbidity and medications | % |
| Donors with diabetes | 52.6 |
| Donors with prior myocardial infarction | 75.1 |
| Donors on angiotensin-converting enzyme inhibitor | 56.2 |
| Donors on beta-blockers | 94 |
| Donors on statins | 90.4 |
| Severity of coronary artery disease | Mean (SD) |
| Prior percutaneous coronary intervention (PCI) | 2.9 (3.1) |
| Prior coronary artery bypass graft (CABG) | 1.3 (0.6) |
| SPECT left ventricular ejection fraction (LVEF) | 60.1 (14) |
| Cell counts and viability | Median (range) |
| Peripheral blood CD34+ cell count/μL | 22.6 (10.7–71.2) |
| Pre-selection total nucleated cell × 108/kg | 3.1 (1.22–6.69) |
| Pre-selection CD34+ cell × 106/kg | 0.79 (0.09–3.53) |
| Pre-selection cell viability (%) | 98 (90–99) |
| Post-selection CD34+ cell (%) | 82.5 (47–95.8) |
| Expression of differentiation markers | Median (range) |
| Post-selection T cells (CD3+) (%) | 1 (0.2–2) |
| Post-selection B cells (CD20+) (%) | 5.5 (2–23) |
| Post-selection platelets/megakaryocytes (CD41+) (%) | 15 (4–81) |
Table II.
Characteristics of subjects enrolled in the ACT34-CMI phase II trial.
| Males and non-pregnant females who are at least 21 years old |
| CCS functional class III or IV chronic refractory angina |
| Attempted and failed ‘best’ medical therapy |
| Identified as unsuitable for conventional revascularization |
| A recent coronary angiogram (within the last 12 months) to document the coronary anatomy and to verify the revascularization procedures |
| Objective evidence using nuclear scan SPECT of inducible ischemia or viable myocardium done during screening |
| A left ventricular ejection fraction ≥25% by ECHO or SPECT |
| Experience at minimum an average of seven angina episodes per week |
| Able to complete a minimum of 3 min but no more than 10 minon a treadmill following the modified Bruce protocol |
| Experience angina or angina-equivalent episodes during the screening exercise treadmill test |
Peripheral blood stem cell collection procedure
Patients were first mobilized with 5 μg/kg G-CSF (Filgrastim) for 5 days. Peripheral CD34+ cells were enumerated on days 4 and 5. Patients’ white blood cells (WBC) were monitored to ensure the WBC were <60 000/mm3 prior to administration of Filgrastim. If a WBC count was ≥60 000/mm3, the principal investigator would order a peripheral blood CD34+ cell count. Based on the results, he would decide to proceed with collection the next day or withhold the Filgrastim injection and repeat the complete blood count (CBC) the next day. Collections were initiated on day 5. Leukapheresis was performed with Amicus (Fenwal, IL, USA). Venous access for all autologous donors was via a tri-lumen central venous catheter. Based on patients’ peripheral CD34+ cell and hematocrit counts, donors were subjected to a two to five blood volume collection that took about 4–5 h to complete. The same machine was used for all collections. However, each donor was assigned randomly to an operator. All operators were specially trained and had demonstrated competency in using this instrument.
CD34+ cell isolation and characteristics
After overnight storage at 4°C, the collected mono-nuclear cells (MNC) were subjected to a CD34+ cell selection procedure using an Isolex 300i Magnetic Cell Selection device (Baxter Healthcare Inc., Deerfield, IL, USA) according to the manufacturer’s instructions.
The median peripheral blood CD34+ cell count on the day of collection was 22.6/μL. The median MNC and CD34+ cell yields were 3.1 × 108/kg and 0.79 × 106/kg, respectively. The cell viability of the collected MNC was above 90%. About 1% of the isolated CD34+ cells expressed CD3, a T-cell antigen, and about 5.5% expressed CD20, a B-cell antigen. There was significant variability in expression of the B-cell antigen ranging from 2% to 23%. In addition, about 15% of the CD34+ cells expressed CD41, a platelet/megakaryocyte antigen (range 4–81%) (Table I). The purity of the isolated cells was tested as outlined below.
Cell freezing and thawing
Cells were resuspended in 1 mL freezing media. Freezing media were prepared with 10% dimethylsulfoxide (DMSO; Sigma-Aldrich, St Louis, MO, USA) and 20% fetal calf serum (FCS; HyClone, Logan, UT, USA) in phosphate-buffered saline (PBS; Invitrogen, Carlsbad, CA, USA). Cells were stored in a −70°C freezer overnight and then transferred into liquid nitrogen and stored for 7 weeks. To thaw the cells, vials were put in a 37°C water bath for 30 s.
Phenotypic characterizations
Flow cytometry
The stem cell product was assayed for the total number of MNC and CD34+ cells. MNC were stained with anti-CD45–fluorescein isothiocyanate (FITC; Pharmingen, San Diego, CA, USA) and anti-CD34–phycoerythrin (PE; Becton Dickinson Immunocytometry Systems, San Jose, CA, USA) and analyzed using a FACSCalibur flow cytometer (BD Biosciences, Waltham, MA, USA).The data analysis was performed using Cell Quest (BD Biosciences) software following International Society of Hematotherapy and Graft engineering (ISHAGE) acquisition layouts. The percentage of CD34+ cells in each sample was determined and the total number of CD34+ cells infused was calculated. CD34 subset (CD133+, CD339+ and CD309+) analysis was performed using a FACSVantage flow cytometer following the ISHAGE protocol (41). Cells were also stained with a T-cell marker (anti-CD3), B-cell marker (anti-CD20) and platelet/megakaryocyte marker (anti-CD41). All antibodies were labeled fluorescently with FITC, PE or Allophycocyanin (APC). For two- or three-color assays, antibodies conjugated with compatible colors were used. Dead cells were excluded from the plots on the basis of 7-Aminoactinomycin D (7-AAD) staining (Becton Dickinson Immunocytometry Systems).
Cytospin slides
Sorted CD34+ cells were stained with anti-CD34–FITC and anti-CD133–PE or anti-CD309–PE antibodies. Cytospin slides were prepared and viewed with a Leica DMLB fluorescent microscope (Leica Microsystems, Wetzlar, Germany). Cytoseal-60 mounting medium (Richard Allen, Kalamazoo, MI, USA) was used. Images were acquired using a SPOT RT color camera (Diagnostic Instruments, Sterling Heights, MI, USA) and processed with SPOT Advanced program version 2.0 (Diagnostic Instruments) and Adobe Photoshop version 6.0 software (Adobe Systems, San Jose, CA, USA).
RNA extraction and quantitative polymerase chain reaction
RNA isolation for examining gene expression by quantitative polymerase chain reaction (qPCR) was performed as described previously (42). Briefly, the quantity of individual mRNA was determined using a two-step quantitative reverse transcriptase (RT)-mediated real-time PCR. RT of total RNA was performed using a high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA, USA). qPCR reactions were performed using 10 ng input cDNA for both target genes and endogenous controls, along with TaqMan Universal PCR master mix (Applied Biosystems). Amplification data were collected using an Applied Biosystems Prism 7900 sequence detector and analyzed using the sequence detection system software (Applied Biosystems). Data were normalized to 18S RNA and CD34 and mRNA abundances were calculated using the ΔΔCT method. qPCR was performed to assess expression levels of CD34, CD133, CD339, VEGFR2, VEGF-A and eNOS. The probe sets and primer pairs for each gene are described in Table III. For the qPCR, normal MNC from good (G) and poor (P) and mobilizer Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as controls.
Table III.
Probe sets and primer pairs for quantitative and qualitative RT-PCR.
| Gene | Probe set | Primer sequence and product size |
|---|---|---|
| CD34 | Applied Biosystems | Forward: 5′-TGA AAA AGC TGG GGA TCC TAG A-3′ |
| Catalog no. 4331182 | Reverse: 5′-TCC CAG GTC CTG AGC TAT AGC C-3′ | |
| Assay ID: Hs00156373_m1 | Product size: 234 | |
| CD133 | Applied Biosystems | Forward: 5′-CAT GGC AAC AGC GAT CAA GG-3′ |
| Catalog no. 4331182 | Reverse: 5′-TTG GAC CAG GCC ATC CAA AT-3′ | |
| Assay ID: Hs01009245_m1 | Product size: 301 | |
| CD339 | Applied Biosystems | Forward: 5′-CGG GAT TTG GTT AAT GGT TAT C-3′ |
| Catalog no. 4331182 | Reverse: 5′-ATA GTC ACT GGC ACG GTT GTA GCA C-3′ | |
| Assay ID: Hs00164982_m1 | Product size: −300 | |
| CD309 | Applied Biosystems | Forward: 5′-ACA AGA CCA AAG GGG CAC GA-3′ |
| Catalog no. 4331182 | Reverse: 5′-GCG CAG GTC CCT GTG GAT AC-3′ | |
| Assay ID: Hs00176676_m1 | Product size: 278 | |
| VEGF-A | Applied Biosystems | Forward: 5′-AAG GAG GAG GGC AGA ATC AT-3′ |
| Catalog no. 4331182 | Reverse: 5′-ATC TGC ATG GTG ATG TTG GA-3′ | |
| Assay ID: Hs00173626_m1 | Product size: 300 | |
| eNOS | Applied Biosystems | Forward: 5′-GCT GCG CCA GGC TCT CAC CTT C-3′ |
| Catalog no. 4331348 | Reverse: 5′-GGC TGC AGC CCT TTG CTC TCA A-3′ | |
| Assay by design | Product size: 300 |
Cell-cycle analysis
The assay involved simultaneous staining of DNA and RNA, which allowed us to distinguish CD34+ cells that were in G0, G1 and S/M states of the cell cycle. Simultaneous staining of DNA and RNA with Hoechst 33342 (Hst) and Pyronin Y allowed further fractionation of the G0/G1 phase. Cell suspensions of 1–2 million cells/mL were made and Hst dye (Molecular Probes, Eugene, OR, USA), to a final concentration of 10 μg/mL, was added and incubated at 37°C for 45 min For staining extracellular markers of HSC, anti-CD34–FITC was added and incubated in the dark for 30 min After staining, cells were suspended in 2 mL 5% paraformaldehyde and kept overnight in a fridge. The next day, Pyronin Y (Sigma, St Louis, MO, USA) was added at a 1:100 dilution and incubated for 30 min at 4°C. Finally, cells were washed once and resuspended in 500 μL 5% paraformaldehyde.
After staining, cells were analyzed using a FACS-Vantage (Becton Dickinson) that was equipped with an argon laser, providing the 488-nm excitation needed for Pyronin Y, and a krypton laser, providing the 350-nm excitation needed for Hst. The Pyronin Y signal was selected with a 575 ± 13-nm bandpass filter and Hst was detected with a 424 ± 22-nm bandpass filter.
Statistical analysis
The linear correlation between two continuous factors was assessed by Spearman’s rank correlation. No statistical adjustment was made for performing multiple tests. All probability values were two-sided and a P-value less than or equal to 0.05 was considered statistically significant.
Results
Assessment of isolated, mobilized peripheral blood CD34+ cells for expression of stem cell-associated markers
CD45 was only used to enumerate CD34. It was not used during CD34 subset analysis. We can only report that the median CD45+ cell percentage was 80% (range 44–95%) and median CD45+ CD34+ cell percentage 83% (range 47–99%).
The median percentage of CD34+ cells that co-expressed CD133 antigen was 71% (range 4.0–89.3%). One patient had significantly low CD133 co-expression (4%); he was a 69-year-old male who weighed 91 kg His total nucleated cell and CD34+ cell counts were 1.36 × 108/kg and 0.17 × 106/kg, respectively. The cell viability was 93%, and 65% of the CD34+ cells from this patient co-expressed CD41, a megakaryocyte/platelet antigen.
The median percentage of CD34+ cells that co-expressed VEGFR2 (CD309) was only 2% (range 0.2–57.1%). The patient with the highest VEGFR2 co-expression (57.1%) was a 55-year-old man who weighed 115 kg. This patient’s total nucleated cell and CD34+ cell counts were 2.86 × 108/kg and 0.81 × 106/kg, respectively. The cell viability was 98%.
The median percentage of CD34+ cells that co-expressed Jagged-1 (CD339) was 3.6% (range 1.0–57.8%). Most patients that had relatively high Jagged-1 co-expression tended to have high VEGFR2 expression (Table IV). The same patient described above had the highest co-expression of VEGFR2 and Jagged-1 antigens. Neither donor sex nor weight appeared to influence the level of expression of these proteins.
Table IV.
Correlation coefficient for isolated CD34+ cell subsets
| CD34 subsets | CD34+ CD133+ | CD34+ CD309+ |
|---|---|---|
| r (P-value) | r (P-value) | |
| CD34+CD133+ | – | 0.4 (0.08) |
| CD34+CD339+ | 0.6 (0.007) | 0.9 (P=0.0001) |
Immunocytochemistry performed on cytospin preparations visualized the phenotypic expression of CD34 and co-expressions of CD133 and VEGFR2 (Figure 1). Figure 2 summarizes the flow cytometric analysis of isolated CD34+ cells that evaluated the co-expression of stem cell-associated proteins. Further studies using qPCR analysis on six patient samples demonstrated the correlation of protein and transcriptome level information (Figure 3).
Figure 1.
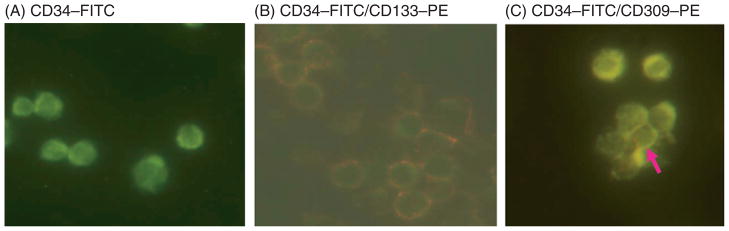
Assessment of co-expression of CD34 antigen with CD133 and VEFR2 antigens by immunocytochemistry. The figure shows cytospins of selected CD34+ cells labeled with (A) anti-CD34–FITC antibodies, with more than 90% of the cells expressing CD34 antigen; (B) anti-CD34–FITC and anti-CD133–PE antibodies, with the majority of the CD34+ cells co-expressing CD133 antigen; and (C) anti-CD34–FITC and anti-CD309–PE (VEGFR2) antibodies, with less than 5% of the CD34+ cells expressing CD309 (arrow). Magnification for all images ×60.
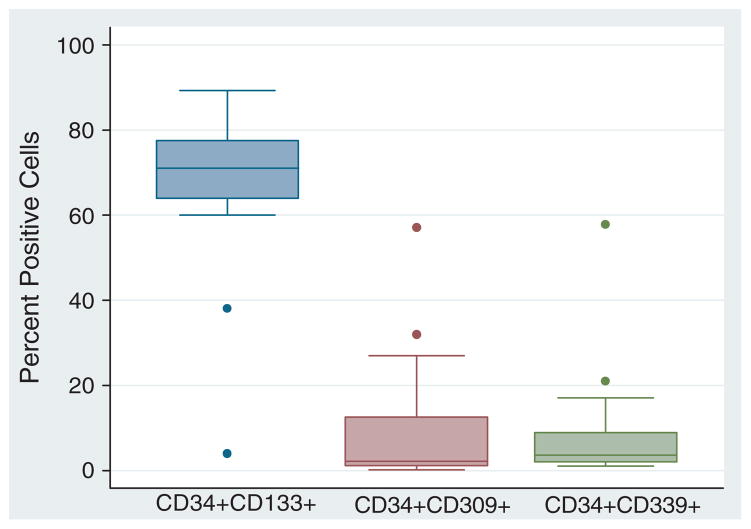
Figure 2.
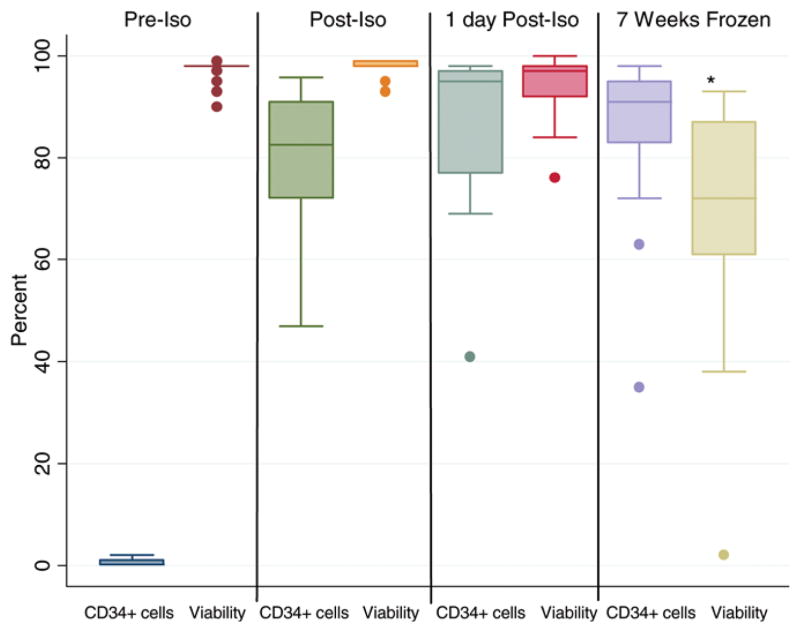
Box-plots showing flow cytometric evaluation of selected CD34+ cells that co-express CD133, CD309 (VEGFR2) and CD339 (Jagged-1). The median percentage purity of the CD34-selected cells was 82.5%; 70% of the cells co-expressed CD133 antigen. Less than 5% of the CD34+ cells co-expressed CD309 and CD339 (n=22).
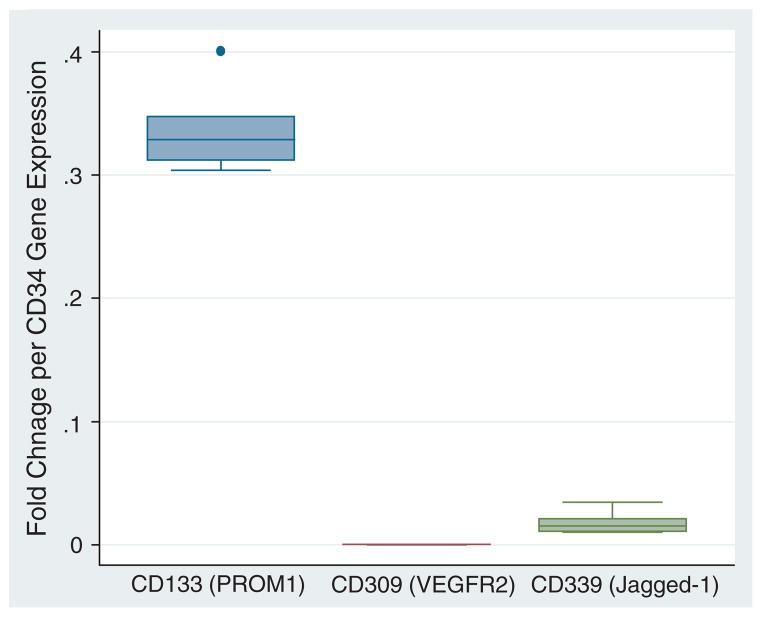
Figure 3.
Quantitative real-time RT-PCR of selected CD34+ cells. Box-plots show gene expression change relative to 18S rRNA expression of CD133, CD309 (VEGFR2) and CD339 (Jagged-1) normalized to CD34 expression. CD34-expressing cells showed significant high co-expression of CD133 compared with Jagged-1 (n=6).
Cell cycle stage: quiescence (G0)
As most stem cells are quiescent, we performed a cell-cycle analysis to determine the cell-cycle status of the isolated CD34+ cells (Figure 4); 78.5% of CD34+ cells were in the G0, quiescent stage (range 3–98%), and only about 6% and 3% of the CD34+ cells were in the G1 and S/M phases, respectively (Figure 5). The patient described above with high VEGFR2 and Jagged-1 receptor co-expression had 27% of the CD34+ cells in S/M phase, the highest for this cohort.
Figure 4.
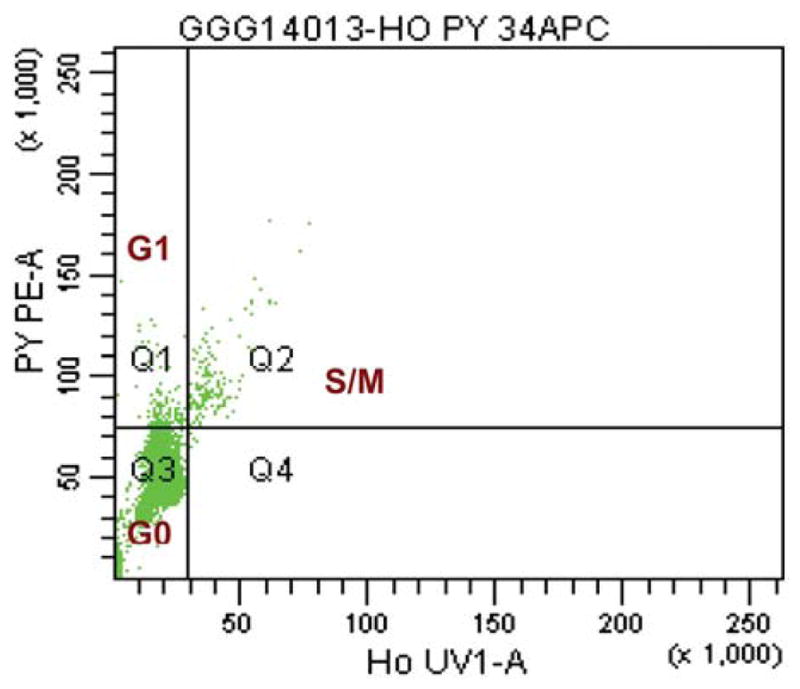
Flow cytometric analysis: figure shows dot-plot of PY (Pyronin Y) on the y-axis and Ho (Hoechst) on the x-axis.
Figure 5.
Cell-cycle analyzes of G-CSF-mobilized CD34-selected cells using an Isolex 3000. The median percentage of CD34+ cells in the G0 stage of the cell cycle was 78.5%.
Assessment of isolated, mobilized peripheral blood CD341 cells for expression of angiogenic factors
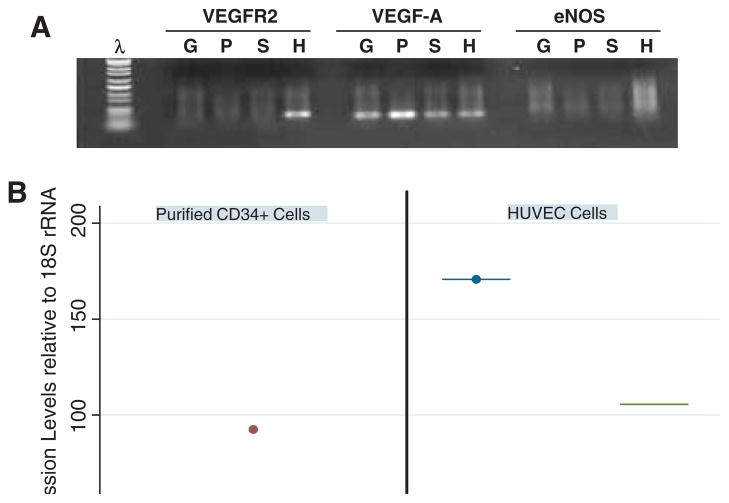
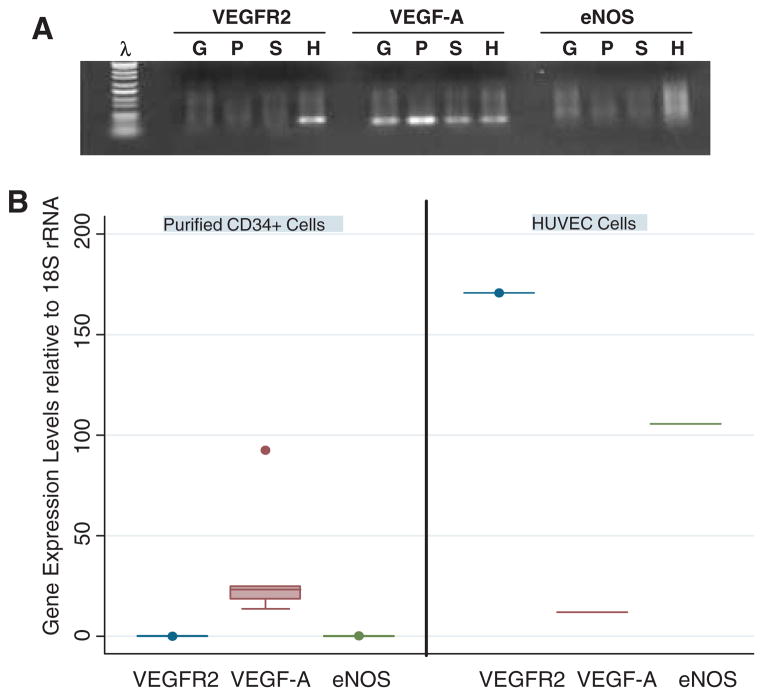
VEGF and nitric oxide release play a critical role in angiogenesis and endothelial function. We therefore assessed the capacity of the isolated CD34+ cells to express VEGF-A and eNOS. Purified CD34+ cells and HUVEC cells as a positive control were subjected to RNA extraction and qPCR (Figure 6). Purified CD34+ cells (S) expressed relatively abundant VEGF-A mRNA but we were unable to detect significant VEGFR2 and eNOS expression at the transcriptome level. Human Umbilical Vein Endothelial Cells (HUVEC) cells (H) and leukapheresis MNC from a donor with good CD34+ cell yield (>5 × 106/kg (G) and another donor with poor CD34+ cell yield (<2 × 106/kg) (P) were used as controls.
Figure 6.
Molecular characterization of G-CSF-mobilized CD34+ cells for VEGFR2, VEGF-A and eNOS. (A) PCR amplification products prepared from MNC (G, P) and sorted CD34+ cells (S). G, sample with good mobilized CD34+ cell yield; P, sample with poor mobilized CD34+ cell yield; H, HUVEC cells, positive control. (B) VEGFR2, VEGF-A and eNOS gene expression relative to 18S rRNA by sorted CD34+ cells from six G-CSF-mobilized patients and HUVEC cells as a control. Both figures show that mobilized MNC and sorted CD34+ cells significantly expressed VEGF-A but not VEGFR2 and eNOS.
Effect of CD34+ selection and storage on CD34+ cell count and viability
The Isolex 300i Magnetic Cell Selection System was used to isolate CD34+ cells positively from leukapheresis products. After the cell-selection procedure, the median percentage of CD34+ cells in the product improved from 0.2% to 80%. There was no significant drop in cell viability after the CD34+ selection procedure. The CD34+ cell content was also not significantly affected by overnight storage at 4°C or freezing for 7 weeks. However, there was a median drop of 26% (P < 0.05) in cell viability after freezing for 7 weeks (Figure 7).
Figure 7.
Impact of CD34+ cell selection and storage on CD34+ cell count and viability. Statistically significant drop in cell viability (P < 0.05). Impact of CD34+ cell selection using an Isolex 300i Magnetic Cell Selection System on the MNC profile.
We assessed the impact of the cell-selection process on total nucleated cell count (TNC) and proportions of T cells, B cells, megakaryocytes, platelets and CD34+ cell subsets in the final cell product. Compared with pre-selection products, there was a statistically significant drop (300-fold drop) in the TNC counts. The median pre-selection TNC count was 32.5 × 109 (range 7–63 × 109) The median post-selection count was 0.1 × 109 (range 0.02–0.4 × 109). The median percentage TNC recovery was only 0.3%. The proportion of T cells in the post-selection products was six-fold lower than the pre-selection percentages. However, the proportion of B cells in the post-selection products significantly increased by about six-fold. Post-selection products contained nearly three times less CD41-expressing cells (megakaryocytes/platelets) and 50% less megakaryocyte progenitors (CD34+ CD41+). The proportion of cells expressing endothelial marker CD309 (VEGFR2/KDR) was significantly depleted, by nearly five-fold, after the Isolex CD34+ selection. In addition, there was 77% drop in the number of cells that expressed prototypic EPC markers (CD34+ VEGFR2+) after the Isolex CD34+ cell enrichment. There was a two-fold increase in CD339 (Jagged-1)-expressing cells, but the proportion of CD34+ cells that co-expressed CD339 significantly dropped from a median of 46% in the pre-selection products to a median of 3.2% in the post-selection products. There was about a 200-fold increase in the proportion of CD133-expressing cells in the post-selection products; however, there was no statistically significant change in the proportion of CD34+ CD133+ cells in post-selection products. This suggested that the Isolex selection process selectively enriched immature CD34+ cells (CD34+ CD133+) and depleted lineage-specific progenitor cells such as EPC (data summarized in Table V).
Table V.
Effect of CD34+ cell selection on MNC characteristics.
| Antigen | Description | Pre-selection | Post-selection | P-value |
|---|---|---|---|---|
| Cell type | Median | Median | ||
| Product volume (mL) | 327 | 100 | ||
| TNC × 106 | 32517 | 108.3 | ||
| CD3+ (%) | T cell | 6 | 1 | <0.01 |
| CD20+ (%) | Early B cell | 1 | 5.5 | <0.01 |
| CD41+ (%) | Megs/platelets | 39 | 15 | 0.39 |
| CD309+ (%) | VEGFR/KDR | 14 | 3 | 0.39 |
| CD339 (%) | Notch/Jagged-1 | 2 | 4 | <0.01 |
| CD133+ (%) | HSC | 0.5 | 51 | <0.01 |
| CD34 subsets | ||||
| CD34+ CD133+ (%) | Immature CD34 | 64 | 71 | 0.31 |
| CD34+ CD309+ (%) | EPC | 79 | 2 | 0.05 |
| CD34+ CD339+ (%) | – | 46 | 3.6 | 0.04 |
| CD34+ CD41+ | Megakaryocyte progenitor | 84 | 30 | 0.02 |
Discussion
In this study we have characterized isolated CD34+ cells from 22 autologous peripheral blood stem cell donors with severe coronary artery disease. Our analysis showed Isolex-selected CD34+ cells had high expression of CD133 antigen. This agrees with a previous report involving healthy G-CSF-mobilized donors that showed 80% of the mobilized CD34+ cells expressed CD133 antigen (43). Primitive HSC are generally thought to be CD34 bright, CD38 dim/−, CD133+ and HLA-DR dim/− (44). It is presumed that primitive HSC contribute primarily to long-term engraftment. Recently, Bonanno et al. (45) suggested that selected CD133+ cells derived from human cord blood differentiated into endothelial and cardiac myocyte-like cells. One report has suggested that endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors but come from CD34+ CD45− cells in the BM and cord blood (46). The CD34+ cells characterized in this study were selected using monoclonal anti-CD34 antibody only. Therefore, our selected cells contained both CD34+ CD45− and CD34+ CD45+ cells.
As 71% of the CD34+ cells in our study co-expressed the primitive CD133 antigen, this suggests our CD34+ cell population consisted of more premature stem cell population than committed EPC. This could be explained by the fact that the majority of the cells are only 3–5 days in peripheral circulation after G-CSF treatment. It is also important to note that all CD34+ subset analyzes were performed after G-CSF treatment, which could have significantly influenced the expression of surface proteins such as VEGFR2 and CXCR4.
One of the principal distinguishing features of HSC, MSC and EPC is VEGFR2 expression (1), as all three of the marrow-derived stem cells have been thought to express CD34 antigen at some stage. Given that our CD34+ cell population may have contained all three of the marrow-derived stem cells, we assessed the proportion of EPC in the Isolex-enriched cells. Our cell analysis showed that less than 2% of the selected CD34+ cells expressed VEGFR2. It has been reported previously that VEGFR2 expression is least in mobilized peripheral blood CD34+ cells, followed by BM CD34+ cells and then cord blood CD34+ cells. Circulating non-mobilized CD34+ cells, even though significantly less in number, have the highest VEGFR2 expression level (43). However, another study by Case et al. (47) has characterized human CD34+ CD133+ VEGFR2+ in peripheral blood, cord blood and mobilized peripheral blood. They reported that mobilized peripheral blood had the highest number of triple positive cells, followed by cord blood and then non-mobilized peripheral blood with the least number.
It is possible that the isolated CD34+ cells in our study were primitive and had not begun expressing VEGFR2 and commitment towards the EPC lineage. It may also be possible that, after exposure to the right environmental cues, they may express VEGFR2 and a full EPC phenotype. Previous studies have shown that CD133+ cells derived from cord blood that initially lacked expression of VEGFR2 became VEGFR-positive after culture with cytokines and growth factors (45). Similarly, BM-derived CD34+ cells were induced to express VEGFR after culture with soluble VEGF (46). Therefore, in the presence of appropriate growth factors and cytokines, CD34+ cells can be induced to differentiate into EPC. In addition, immobilized platelets have been shown to recruit CD34+ cells and induce their differentiation into mature endothelial cells (48,49). Further studies will be needed to account for the high VEGFR2 co-expression by CD34+ cells in a few patients. However, we were unable to confirm this observation by molecular studies. It is also unknown whether CD34+ cells from these high VEGFR2 expressers have a superior capacity to induce angiogenesis and could therefore help those patients achieve a better clinical outcome.
Our observation that the enriched CD34+ cells show relatively high expression of VEGF-A suggests that this could be the mechanism by which CD34+ cells induce angiogenesis. These findings corroborate previous reports that suggest isolated CD34+ cells from peripheral blood, cord blood and BM express significantly high VEGF-A and very poor VEGFR2 expression (24,50). A small number of labeled CD34+ cells have been observed at the site of angiogenesis in animal models (51,52), suggesting that CD34+ cell release of VEGF-A represents a potential important mechanism for recruitment and proliferation of EPC, and has a role in creating a pro-angiogenic environment. Previous attempts to induce angiogenesis by injecting soluble VEGF showed conflicting results and further studies are needed (53–55). The lack of a steady and prolonged supply of VEGF-A, as may be achieved by implanted CD34+ cells, may explain the failures in some of these experiments. Other non-CD34+ cells can produce VEGF, as we show in Figure 6A, with mobilized MNC expressing VEGF-A. We found that poor mobilizers express higher levels of VEGF-A compared with good mobilizers (data not shown). This raises the question of whether other mobilized non-CD34+ cells can be equally effective in inducing angiogenesis, and questions the validity of CD34+ cell selection. However, one report by Kawamoto et al. (56) seems to suggest injection of purified CD34+ cells is more effective in inducing angiogenesis than total MNC.
Our analysis showed that less than 5% of the isolated CD34+ cells expressed Jagged-1 protein. This suggests notch signaling activity is very low in the isolated CD34+ cells. It has been suggested that notch signaling promotes epithelial-to-mesenchymal transformation and plays a role in heart and vessel development (57). It has been shown previously that the interaction of tumor necrosis factor 1a and lipopolysaccharide with EPC in the BM microenvironment modulates notch signaling (58). It is therefore possible that the lack of notch signaling activity in our isolated CD34+ cells is only transient. Further studies will be needed to determine whether injection of CD34+ cells in ischemic myocardium induces higher expression of Jagged-1 and higher notch signaling activities.
Cell-cycle analysis of the selected CD34+ cells showed that greater than 80% of the cells were quiescent. This is in agreement with previous reports (59–62). However, EPC can be induced to proliferate, differentiate and form new vessels in response to laminar shear stress (63). It has been shown previously, after transplantation, that these quiescent EPC proliferate in vivo and participate in angiogenesis and vasculogenesis (64). This supports our observation that patients with a high proportion of proliferating CD34+ cells tend to express high Jagged-1 and VEGFR2.
Our study shows that overnight storage of the isolated CD34+ cells, which were suspended in autologous plasma, did not affect significantly their number or viability. However, freezing in 10% DMSO for 7 weeks significantly affected cell viability. Controlled-rate freezing was not used to freeze the cells and we suspect that this resulted in water crystallization and cell damage during thawing. In our hands, cell viability of thawed HPC for BM transplant that had been frozen using controlled rate freezing is usually in the range of 90–95% (data not shown).
It has long been known that there are differences among the various devices and immunomagnetic antibodies used to select CD34+ cells (65–67). However, most published studies focus on differences in purity and cell viability. We could not find any study that compared the proportions of CD34 subsets in the final products after enrichment with various cell-selection devices and antibodies. The most common devices used to isolate CD34+ cells for clinical applications are the Isolex 300i Magnetic Cell Selection System, CellPro CEPRATE and Miltenyi CliniMACS. Among the three, only the Isolex 300i Magnetic Cell Selection System is Food and Drug Administration (FDA) approved for clinical use. Our study has demonstrated that using the Isolex 300i Magnetic Cell Selection System to select CD34+ cells results in final products that contain significantly less lineage-specific progenitor cells such as EPC and megakaryocyte progenitors. In addition, accessory cells such as T cells, which are known to play a role in cell homing, were significantly depleted in the final products. However, B cells appeared to be significantly enriched. Putting all this together, it is very likely that these differences in proportions of pre- and post-isolation CD34 subsets, if variable among the various methods used to purify CD34+ cells, may contribute to the variability in clinical outcomes observed in many cell-therapy clinical trials.
In summary, we have characterized mobilized peripheral blood-derived CD34+ cells from patients with severe coronary artery disease and evaluated their potential as a source of EPC. The isolated CD34+ cells have all the characteristics of immature and quiescent stem cells that lack expression of specific endothelial-related markers. Enriched CD34+ cells showed a relatively abundant expression of VEGF-A and this may be one of the mechanisms by which CD34+ cells facilitate angiogenesis.
Acknowledgments
Supported by NIH grants CA102824 and a grant from Baxter Healthcare Inc.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–8. [PubMed] [Google Scholar]
- 2.Verfaillie CM, Pera MF, Lansdorp PM. Stem cells: hype and reality. Hematology (Am Soc Hematol Educ Program) 2002:369–91. doi: 10.1182/asheducation-2002.1.369. [DOI] [PubMed] [Google Scholar]
- 3.Zubair AC, Silberstein L, Ritz J. Adult hematopoietic stem cell plasticity. Transfusion. 2002;42:1096–101. doi: 10.1046/j.1537-2995.2002.00156.x. [DOI] [PubMed] [Google Scholar]
- 4.Eichmann A, Corbel C, Nataf V, Vaigot P, Breant C, Le Douarin NM. Ligand-dependent development of the endothelial and hemopoietic lineages from embryonic mesodermal cells expressing vascular endothelial growth factor receptor 2. Proc Natl Acad Sci, USA (PNAS) 1997;94:5141–6. doi: 10.1073/pnas.94.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–32. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 6.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 7.George J, Shmilovich H, Deutsch V, Miller H, Keren G, Roth A. Comparative analysis of methods for assessment of circulating endothelial progenitor cells. Tissue Eng. 2006;12:331–5. doi: 10.1089/ten.2006.12.331. [DOI] [PubMed] [Google Scholar]
- 8.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–12. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 9.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscl Thromb Vasc Biol. 2003;23:1185–9. doi: 10.1161/01.ATV.0000073832.49290.B5. [DOI] [PubMed] [Google Scholar]
- 10.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 11.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 12.Simper D, Wang S, Deb A, Holmes D, McGregor C, Frantz R, et al. Endothelial progenitor cells are decreased in blood of cardiac allograft patients with vasculopathy and endothelial cells of noncardiac origin are enriched in transplant atherosclerosis. Circulation. 2003;108:143–9. doi: 10.1161/01.CIR.0000081703.34526.5D. [DOI] [PubMed] [Google Scholar]
- 13.George J, Herz I, Goldstein E, Abashidze S, Deutch V, Finkelstein A, et al. Number and adhesive properties of circulating endothelial progenitor cells in patients with instent restenosis. Arterioscl Throm Vasc Biol. 2003;23:E57–60. doi: 10.1161/01.ATV.0000107029.65274.db. [DOI] [PubMed] [Google Scholar]
- 14.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–6. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–8. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 16.Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–9. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 17.Gill M, Dias S, Hattori K, Rivera ML, Hicklin D, Witte L, et al. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res. 2001;88:167–74. doi: 10.1161/01.res.88.2.167. [DOI] [PubMed] [Google Scholar]
- 18.George J, Goldstein E, Abashidze S, Deutsch V, Shmilovich H, Finkelstein A, et al. Circulating endothelial progenitor cells in patients with unstable angina: association with systemic inflammation. Eur Heart J. 2004;25:1003–8. doi: 10.1016/j.ehj.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 19.Llevadot J, Murasawa S, Kureishi Y, Uchida S, Masuda H, Kawamoto A, et al. HMG-CoA reductase inhibitor mobilizes bone marrow-derived endothelial progenitor cells. J Clin Invest. 2001;108:399–405. doi: 10.1172/JCI13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, et al. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–90. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 21.Kalka C, Masuda H, Takahashi T, Gordon R, Tepper O, Gravereaux E, et al. Vascular endothelial growth factor(165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res. 2000;86:1198–202. doi: 10.1161/01.res.86.12.1198. [DOI] [PubMed] [Google Scholar]
- 22.Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer. 2008;8:880–7. doi: 10.1038/nrc2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klagsbrun M, D’Amore PA. Vascular endothelial growth factor and its receptors. Cyto Grow Fact Rev. 1996;7:259–70. doi: 10.1016/s1359-6101(96)00027-5. [DOI] [PubMed] [Google Scholar]
- 24.Serefhanoglu S, Goker H, Buyukasik Y, et al. Changes in vascular endothelial growth factor, angiopoietins, and Tie-2 levels with G-CSF stimulation in healthy donors. Ann Hematol. 2009;88:667–671. doi: 10.1007/s00277-008-0657-7. [DOI] [PubMed] [Google Scholar]
- 25.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–95. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 26.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 27.Ortega N, Jonca F, Vincent S, Favard C, Ruchoux MM, Plouet J. Systemic activation of the vascular endothelial growth factor receptor KDR/flk-1 selectively triggers endothelial cells with an angiogenic phenotype. Am J Pathol. 1997;151:1215–24. [PMC free article] [PubMed] [Google Scholar]
- 28.Gallacher L, Murdoch B, Wu DM, Karanu FN, Keeney M, Bhatia M. Isolation and characterization of human CD34(−) Lin(−) and CD34(+)Lin(−) hematopoietic stem cells using cell surface markers AC133 and CD7. Blood. 2000;95:2813–20. [PubMed] [Google Scholar]
- 29.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–12. [PubMed] [Google Scholar]
- 30.Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106–12. [PubMed] [Google Scholar]
- 31.Gering M, Patient R. Notch in the niche. Cell Stem cell. 2008;2:293–4. doi: 10.1016/j.stem.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 32.Ahmad S, Hewett PW, Wang P, Al-Ani B, Cudmore M, Fujisawa T, et al. Direct evidence for endothelial vascular endothelial growth factor receptor-1 function in nitric oxide-mediated angiogenesis. Circ Res. 2006;99:715–22. doi: 10.1161/01.RES.0000243989.46006.b9. [DOI] [PubMed] [Google Scholar]
- 33.Morbidelli L, Donnini S, Ziche M. Role of nitric oxide in the modulation of angiogenesis. Curr Pharm Des. 2003;9:521–30. doi: 10.2174/1381612033391405. [DOI] [PubMed] [Google Scholar]
- 34.Kondo T, Kobayashi K, Murohara T. Nitric oxide signaling during myocardial angiogenesis. Mol Cell Biochem. 2004;264:25–34. doi: 10.1023/b:mcbi.0000044371.06317.0a. [DOI] [PubMed] [Google Scholar]
- 35.Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet. 2008;9:115–28. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- 36.Plett PA, Frankovitz SM, Orschell-Traycoff CM. In vivo trafficking, cell cycle activity, and engraftment potential of phenotypically defined primitive hematopoietic cells after transplantation into irradiated or nonirradiated recipients. Blood. 2002;100:3545–52. doi: 10.1182/blood.V100.10.3545. [DOI] [PubMed] [Google Scholar]
- 37.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–94. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 38.Jin F, Zhai Q, Qiu L, Meng H, Zou D, Wang Y, et al. Degradation of BM SDF-1 by MMP-9: the role in G-CSF-induced hematopoietic stem/progenitor cell mobilization. Bone Marrow Transplant. 2008;42:581–8. doi: 10.1038/bmt.2008.222. [DOI] [PubMed] [Google Scholar]
- 39.Kuethe F, Krack A, Fritzenwanger M, Herzau M, Opfermann T, Pachmann K, et al. Treatment with granulocyte-colony stimulating factor in patients with acute myocardial infarction. Evidence for a stimulation of neovascularization and improvement of myocardial perfusion. Die Pharmazie. 2006;61:957–61. [PubMed] [Google Scholar]
- 40.Ohki Y, Heissig B, Sato Y, Akiyama H, Zhu Z, Hicklin DJ, et al. Granulocyte colony-stimulating factor promotes neovascularization by releasing vascular endothelial growth factor from neutrophils. FASEB J. 2005;19:2005–7. doi: 10.1096/fj.04-3496fje. [DOI] [PubMed] [Google Scholar]
- 41.Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996;5:213–26. doi: 10.1089/scd.1.1996.5.213. [DOI] [PubMed] [Google Scholar]
- 42.Chen L, Necela BM, Su W, Yanagisawa M, Anastasiadis PZ, Fields AP, et al. Peroxisome proliferator-activated receptor gamma promotes epithelial to mesenchymal transformation by Rho GTPase-dependent activation of ERK1/2. J Biol Chem. 2006;281:24575–87. doi: 10.1074/jbc.M604147200. [DOI] [PubMed] [Google Scholar]
- 43.Tura O, Barclay GR, Roddie H, Davies J, Turner ML. Absence of a relationship between immunophenotypic and colony enumeration analysis of endothelial progenitor cells in clinical haematopoietic cell sources. J Transl Med. 2007;5:37. doi: 10.1186/1479-5876-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baum CM, Weissman IL, Tsukamoto AS, Buckle AM, Peault B. Isolation of a candidate human hematopoietic stem-cell population. Proc Natl Acad Sci USA. 1992;89:2804–8. doi: 10.1073/pnas.89.7.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonanno G, Mariotti A, Procoli A, Corallo M, Rutella S, Pessina G, et al. Human cord blood CD133+ cells immunoselected by a clinical-grade apparatus differentiate in vitro into endothelial- and cardiomyocyte-like cells. Transfusion. 2007;47:280–9. doi: 10.1111/j.1537-2995.2007.01104.x. [DOI] [PubMed] [Google Scholar]
- 46.Timmermans F, Van Hauwermeiren F, De Smedt M, Raedt R, Plasschaert F, De Buyzere ML, et al. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscl Thromb Vasc Biol. 2007;27:1572–9. doi: 10.1161/ATVBAHA.107.144972. [DOI] [PubMed] [Google Scholar]
- 47.Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, et al. Human CD34+AC133+ VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35:1109–18. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Daub K, Langer H, Seizer P, Stellos K, May AE, Goyal P, et al. Platelets induce differentiation of human CD34+ progenitor cells into foam cells and endothelial cells. FASEB J. 2006;20:2559–61. doi: 10.1096/fj.06-6265fje. [DOI] [PubMed] [Google Scholar]
- 49.Langer H, May AE, Daub K, Heinzmann U, Lang P, Schumm M, et al. Adherent platelets recruit and induce differentiation of murine embryonic endothelial progenitor cells to mature endothelial cells in vitro. Circ Res. 2006;98:E2–10. doi: 10.1161/01.RES.0000201285.87524.9e. [DOI] [PubMed] [Google Scholar]
- 50.Pomyje J, Zivny J, Sefc L, Plasilova M, Pytlik R, Necas E. Expression of genes regulating angiogenesis in human circulating hematopoietic cord blood CD34+/CD133+ cells. Eur J Haematol. 2003;70:143–50. doi: 10.1034/j.1600-0609.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- 51.Popa ER, Harmsen MC, Tio RA, van der Strate BW, Brouwer LA, Schipper M, et al. Circulating CD34+ progenitor cells modulate host angiogenesis and inflammation in vivo. J Mol Cell Cardiol. 2006;41:86–96. doi: 10.1016/j.yjmcc.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 52.Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–8. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bougioukas I, Didilis V, Ypsilantis P, Giatromanolaki A, Sivridis E, Lialiaris T, et al. Intramyocardial injection of low-dose basic fibroblast growth factor or vascular endothelial growth factor induces angiogenesis in the infarcted rabbit myocardium. Cardiovasc Pathol. 2007;16:63–8. doi: 10.1016/j.carpath.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 54.Ripa RS, Wang Y, Jorgensen E, Johnsen HE, Hesse B, Kastrup J. Intramyocardial injection of vascular endothelial growth factor-A165 plasmid followed by granulocyte-colony stimulating factor to induce angiogenesis in patients with severe chronic ischaemic heart disease. Eur Heart J. 2006;27:1785–92. doi: 10.1093/eurheartj/ehl117. [DOI] [PubMed] [Google Scholar]
- 55.Schwarz ER, Speakman MT, Patterson M, Hale SS, Isner JM, Kedes LH, et al. Evaluation of the effects of intramyocardial injection of DNA expressing vascular endothelial growth factor (VEGF) in a myocardial infarction model in the rat: angiogenesis and angioma formation. J Am Coll Cardiol. 2000;35:1323–30. doi: 10.1016/s0735-1097(00)00522-2. [DOI] [PubMed] [Google Scholar]
- 56.Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, et al. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–9. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 57.Koyanagi M, Bushoven P, Iwasaki M, Urbich C, Zeiher AM, Dimmeler S. Notch signaling contributes to the expression of cardiac markers in human circulating progenitor cells. Circ Res. 2007;101:1139–45. doi: 10.1161/CIRCRESAHA.107.151381. [DOI] [PubMed] [Google Scholar]
- 58.Fernandez L, Rodriguez S, Huang H, Chora A, Fernandes J, Mumaw C, et al. Tumor necrosis factor-alpha and endothelial cells modulate Notch signaling in the bone marrow microenvironment during inflammation. Exp Hematol. 2008;36:545–58. doi: 10.1016/j.exphem.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paz H, Wong CA, Li W, Santat L, Wong KK, Chatterjee S. Quiescent subpopulations of human CD34-positive hematopoietic stem cells are preferred targets for stable recombinant adeno-associated virus type 2 transduction. Hum Gene Ther. 2007;18:614–26. doi: 10.1089/hum.2006.188. [DOI] [PubMed] [Google Scholar]
- 60.Fukuda S, Pelus LM. Elevation of Survivin levels by hematopoietic growth factors occurs in quiescent CD34+ hematopoietic stem and progenitor cells before cell cycle entry. Cell Cycle (TX) 2002;1:322–6. [PubMed] [Google Scholar]
- 61.Jetmore A, Plett PA, Tong X, Wolber FM, Breese R, Abonour R, et al. Homing efficiency, cell cycle kinetics, and survival of quiescent and cycling human CD34(+) cells transplanted into conditioned NOD/SCID recipients. Blood. 2002;99:1585–93. doi: 10.1182/blood.v99.5.1585. [DOI] [PubMed] [Google Scholar]
- 62.Shah AJ, Smogorzewska EM, Hannum C, Crooks GM. Flt3 ligand induces proliferation of quiescent human bone marrow CD34+CD38− cells and maintains progenitor cells in vitro. Blood. 1996;87:3563–70. [PubMed] [Google Scholar]
- 63.Yamamoto K, Takahashi T, Asahara T, Ohura N, Sokabe T, Kamiya A, et al. Proliferation, differentiation, and tube formation by endothelial progenitor cells in response to shear stress. J Appl Physiol. 2003;95:2081–8. doi: 10.1152/japplphysiol.00232.2003. [DOI] [PubMed] [Google Scholar]
- 64.Murayama T, Tepper OM, Silver M, Ma H, Losordo DW, Isner JM, et al. Determination of bone marrow-derived endothelial progenitor cell significance in angiogenic growth factor-induced neovascularization in vivo. Exp Hematol. 2002;30:967–72. doi: 10.1016/s0301-472x(02)00867-6. [DOI] [PubMed] [Google Scholar]
- 65.O’Donnell PV, Myers B, Edwards J, Loper K, Rhubart P, Noga SJ. CD34 selection using three immunoselection devices: comparison of T-cell depleted allografts. Cytotherapy. 2001;3:483–8. doi: 10.1080/146532401317248081. [DOI] [PubMed] [Google Scholar]
- 66.McNiece IK, Stoney GB, Kern BP, Briddell RA. CD34+ cell selection from frozen cord blood products using the Isolex 300i and CliniMACS CD34 selection devices. J Hematother. 1998;7:457–61. doi: 10.1089/scd.1.1998.7.457. [DOI] [PubMed] [Google Scholar]
- 67.Watts MJ, Somervaille TC, Ings SJ, Ahmed F, Khwaja A, Yong K, et al. Variable product purity and functional capacity after CD34 selection: a direct comparison of the CliniMACS (v2.1) and Isolex 300i (v2.5) clinical scale devices. Br J Haematol. 2002;118:117–23. doi: 10.1046/j.1365-2141.2002.03561.x. [DOI] [PubMed] [Google Scholar]