Fig. 1.
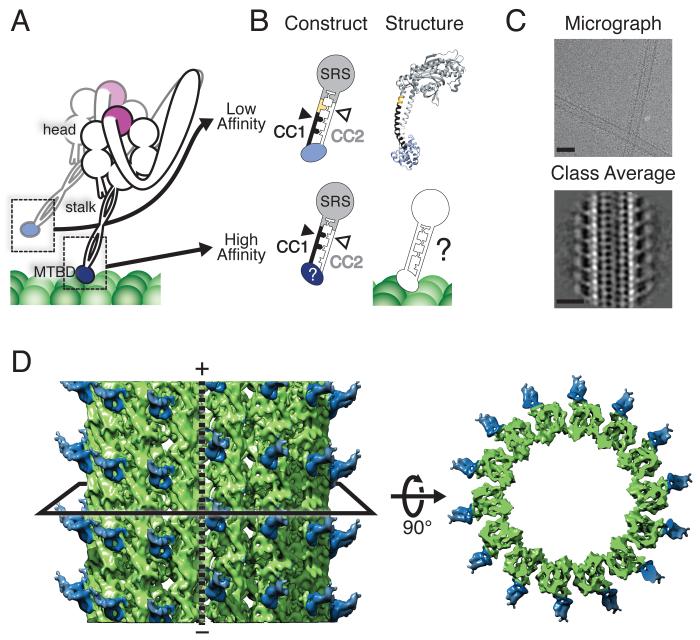
Cryo-EM reconstruction of the cytoplasmic dynein high-affinity MTBD bound to a MT. (A) Schematic of dimeric cytoplasmic dynein. Major features relevant to this study are indicated. The MTBD is depicted in its low- (light blue) and high- (dark blue) affinity states during a step along the MT. (B) Schematic of the fusion constructs between the MTBD and seryl-tRNA synthetase (SRS) that fix the heptad registry of the stalk. The low-affinity construct has an additional 4 amino acids (yellow) inserted in CC1 (black) relative to the high-affinity construct. (C) Cryo-EM image of MTs highly decorated with the high-affinity SRS-MTBD construct (top) and a class average generated from segments of decorated 14-protofilament MTs (bottom). Scale bars: 25 nm. (D) Three-dimensional reconstruction of the MTBD-MT complex, filtered to the calculated resolution of 9.7 Å (Fig. S1). The black solid line represents a slice through the volume, which is shown on the right viewed from the minus end of the MT. The MT polarity is indicated and the dashed line shows the location of the MT seam. The SRS has been omitted due to its lower resolution (Fig. S1).