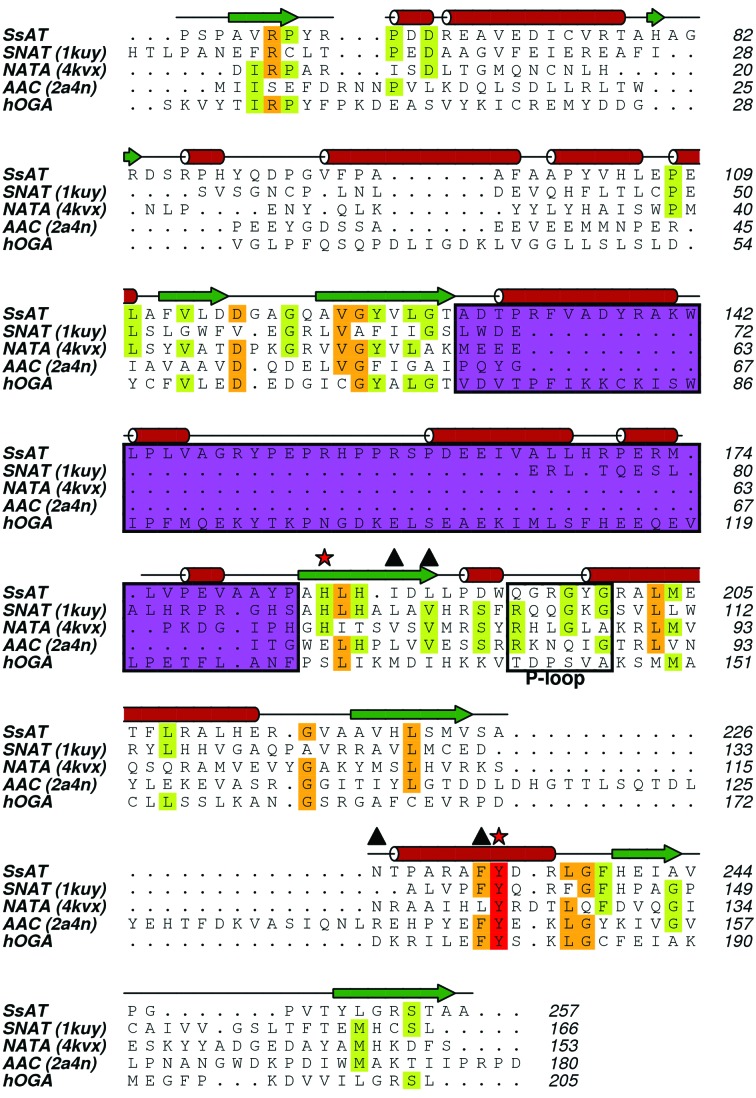
Figure 5.
Multi-sequence alignment of SsAT, hOGA and several GNATs referenced in the text. Secondary-structural elements are highlighted, with α-helices as cylinders and β-strands as arrows, based on the secondary-structure assignment of SsAT. The P-loop responsible for the binding of the pyrophosphate moiety of AcCoA is highlighted in a box. Note that hOGA shows an ‘atypical’ sequence in this region. A tyrosine and a histidine (marked with red stars) have been proposed to act as catalytic residues. Several residues, besides the P-loop, that interact with AcCoA are marked with triangles. The region between β3 and β4 coloured in purple shows a variable length in different acetyltransferase structures; SsAT and hOGA both have unique extensive insertions here.