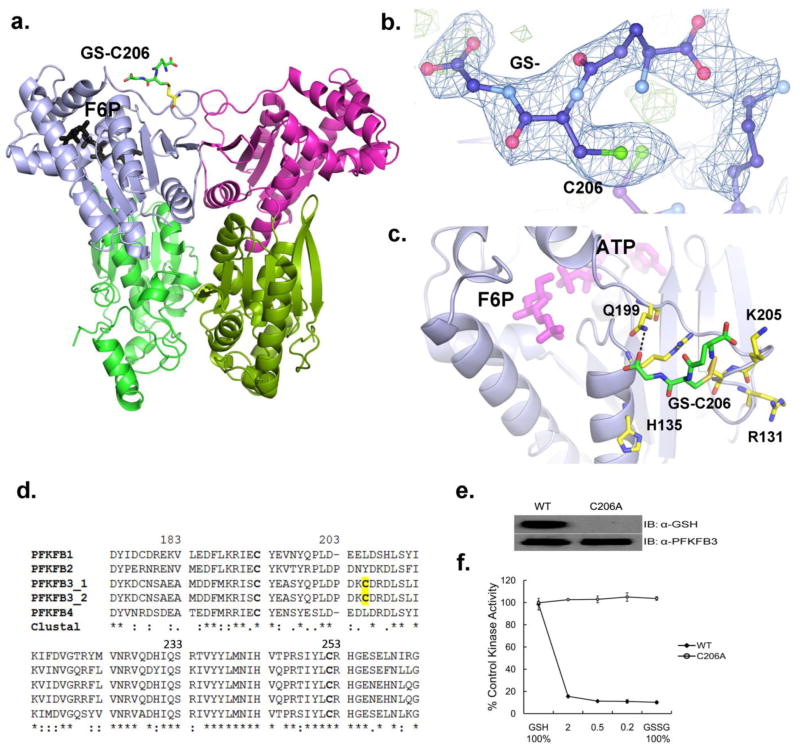
Figure 2. Position of S-glutathionylation in PFKFB3.
(a) A ribbon diagram of the PFKFB3 dimer showing site of S-glutathionylation. S-glutathionylation is shown in only one subunit for a clearer view. The two catalytic domains are shown in different colors: the 2-Kinase domains, gray and magenta; the 2-Phosphatase domains, light green and dark green. S-glutathionylated C206 is labeled as GS-C206 and the position of Fru-6-P in the 2-Kinase catalytic pocket is also shown. (b) The glutathione moiety (labeled as GS-) bound to C206 is shown with an |Fo|–|Fc| omit map at a 2.5σ level. (c) Glutathione (GS-) bound to Cys206 is located on the kinase surface surrounded by basic residues, Arg131, His135, and Lys205. Glutathione has a hydrogen bond with Gln199. The protein residues are shown in yellow, glutathione in green, and the Fru-6-P and ADP in magenta for comparison. (d) Amino acid sequence alignment among human PFKFB isoforms, PFKFB1, PFKFB2, Two splicing variants of PFKFB3, and PFKFB4 from the ClustalW program. Fully conserved, conserved, and similar residues at each position are symbolized by an asterisk (*), colon (:), and dot (.), respectively. The conserved cysteine residues are in bold type and yellow shading shows PFKFB3 isoform-specific S-glutathionylated cysteine residue. (e) Immunoblotting of WT-PFKFB3 and the C206A mutant after pre-treatment with 3 mM 1:1 molar ratio of GSH to GSSG. (f) The 2-Kinase activities of WT-PFKFB3 and the C206A mutant after 25 min pre-treatment with varied molar ratio of GSH to GSSG.