Abstract
Francisella tularensis is a highly infectious Gram-negative pathogen that replicates intracellularly within the mammalian host. One of the factors associated with virulence of F. tularensis is the protein FupA that mediates high-affinity transport of ferrous iron across the outer membrane. Together with its paralogue FslE, a siderophore–ferric iron transporter, FupA supports survival of the pathogen in the host by providing access to the essential nutrient iron. The FupA orthologue in the attenuated live vaccine strain (LVS) is encoded by the hybrid gene fupA/B, the product of an intergenic recombination event that significantly contributes to attenuation of the strain. We used 55Fe transport assays with mutant strains complemented with the different paralogues to show that the FupA/B protein of LVS retains the capacity for high-affinity transport of ferrous iron, albeit less efficiently than FupA of virulent strain Schu S4. 55Fe transport assays using purified siderophore and siderophore-dependent growth assays on iron-limiting agar confirmed previous findings that FupA/B also contributes to siderophore-mediated ferric iron uptake. These assays further demonstrated that the LVS FslE protein is a weaker siderophore–ferric iron transporter than the orthologue from Schu S4, and may be a result of the sequence variation between the two proteins. Our results indicate that iron-uptake mechanisms in LVS differ from those in Schu S4 and that functional differences in the outer membrane iron transporters have distinct effects on growth under iron limitation.
Introduction
The Gram-negative bacterium Francisella tularensis is the causative agent of the zoonotic disease tularaemia (Sjöstedt, 2007). Strains of F. tularensis subsp. tularensis are restricted to North America and induce a more severe form of disease than strains of the more widespread F. tularensis subsp. holarctica. The highly infectious pathogen can access the mammalian host by multiple routes: by aerosol, entry through cuts or wounds or by the gastrointestinal route. The live vaccine strain (LVS) is an attenuated F. tularensis subsp. holarctica derivative that can provide limited protection against virulent type A strains (Burke, 1977). Although highly attenuated by the intradermal or subcutaneous route of infection in mice, LVS is able to cause a fatal infection in mice at low dose when introduced by the intraperitoneal route (Fortier et al., 1991), and can invade and replicate within isolated mammalian cells.
Genome sequencing and molecular analyses have identified several differences between LVS and virulent strains that may contribute to virulence differences (Rohmer et al., 2006; Salomonsson et al., 2009; Svensson et al., 2005). One such difference rests with neighbouring paralogous genes fupA and fupB of the virulent Francisella species, which in LVS have undergone a recombination event leading to formation of the unique fupA/B hybrid gene (Rohmer et al., 2006). Introduction of the fupA gene from a virulent strain into LVS was shown to significantly enhance virulence following subcutaneous infection of mice (Salomonsson et al., 2009). Spontaneous generation of a similar fusion protein also led to attenuation in a strain of F. tularensis subsp. tularensis, as did targeted deletion of fupA (Twine et al., 2005). Based on these findings, the FupA/B fusion protein is predicted to have altered function compared with the intact FupA.
FupA is an outer membrane protein that belongs to a protein family unique to Francisella species (Larsson et al., 2005) and is associated with acquisition of the essential nutrient iron in F. tularensis subsp. tularensis strain Schu S4 (Lindgren et al., 2009; Ramakrishnan et al., 2012). Genetic analysis and 55Fe transport assays in Schu S4 demonstrated that FupA mediates high-affinity transport of ferrous iron (Ramakrishnan et al., 2008, 2012). FupB, encoded by the gene adjacent to fupA, is not important for ferrous iron transport (Ramakrishnan et al., 2012). The FupA/B protein of LVS has the amino-terminal 297 aa residues of FupA fused to the carboxy-terminal 241 residues of FupB at a central conserved stretch of 12 aa. This fusion protein might therefore be expected to be defective in ferrous iron transport.
FslE, a paralogue of FupA, was shown to mediate siderophore-dependent iron acquisition in Schu S4 using growth assays on iron-limiting agar, and 55Fe uptake assays subsequently confirmed its role as siderophore transporter (Ramakrishnan et al., 2008, 2012). FslE is encoded within the fsl siderophore operon, which is conserved and is similarly expressed in different Francisella strains including LVS and Schu S4 (Sullivan et al., 2006; Deng et al., 2006; Milne et al., 2007). Despite similarities in siderophore production, siderophore utilization assays indicated that LVS differs in siderophore utilization; FslELVS appeared to be unimportant for siderophore-dependent growth promotion on agar and, rather paradoxically, FupA/B seemed to be more critical in these assays (Sen et al., 2010). The current study aimed at characterizing iron acquisition in LVS and determining how the involvement of FupA/B and FslE in these processes differs from that of their orthologues in virulent Schu S4. Our data indicate that FupA/B functions both in siderophore-iron and in ferrous iron acquisition, thereby playing a dominant role in iron acquisition by LVS.
Methods
Bacterial strains and culture.
F. tularensis subsp. holarctica and F. tularensis subsp. tularensis strains used in this study are listed in Table 1. Strains were maintained on modified Mueller–Hinton agar supplemented with serum, cysteine and iron salts (MHA). Chamberlain’s defined medium (CDM) (Chamberlain, 1965) was used for routine liquid culture of strains. Iron-limiting agar plates used CDM without addition of iron salts (CDM-Fe) (Sullivan et al., 2006). Chelex-treated CDM (che-CDM) was supplemented with MgSO4 and CaCl2 and defined levels of ferric pyrophosphate (FePPi) (2.5 µg ml−1 for iron-replete and 0.125 µg ml−1 for iron-limiting medium) (Ramakrishnan et al., 2012). Escherichia coli strain MC1061.1 (araD139 Δ(ara-leu)7696 galE15 galK16 Δ(lac)X74 rpsL (Strr) hsdR2 (rK–mK+) mcrA mcrB1 recA) was grown in Luria broth and used for routine cloning. Ampicillin for E. coli transformants was used at 50 µg ml−1 (for liquid culture) or at 100 µg ml−1 (agar plates). Kanamycin at 15 µg ml−1 was used to select for plasmid integrants in F. tularensis.
Table 1. Strains used in this study.
| Strain | Description | Source or reference |
| F. tularensis | ||
| LVS | F. tularensis subsp. holarctica LVS | K. Elkins |
| GR7 | LVS ΔfslA | Sullivan et al. (2006) |
| GR13 | LVS ΔfslE | Sen et al. (2010) |
| GR16 | LVS ΔfupA/B | Sen et al. (2010) |
| GR17 | LVS ΔfslE ΔfupA/B | Sen et al. (2010) |
| GR20 | GR16 (pGIR459); vector integrant | Sen et al. (2010) |
| GR21 | GR17 (pGIR459); vector integrant | Sen et al. (2010) |
| GR23 | GR17 (pGIR474); fslESchu plasmid integrant | Sen et al. (2010) |
| GR24 | GR16 (pGIR479); fupA/B plasmid integrant | Sen et al. (2010) |
| GR25 | GR17 (pGIR479); fupA/B plasmid integrant | Sen et al. (2010) |
| GR34 | GR16 (pGIR477); fupA plasmid integrant | This study |
| GR36 | GR17 (pGIR477); fupA plasmid integrant | This study |
| GR38 | GR17 (pBAS3); fupA+fupB plasmid integrant | This study |
| GR39 | GR17 (pBAS3); fupA+fupB plasmid integrant | This study |
| GR68 | GR17 (pSC1); fslELVS plasmid integrant | This study |
| GR215 | Schu S4 (pGIR459); vector integrant | Ramakrishnan et al. (2012) |
| GR219 | Schu S4 ΔfslE ΔfupA | Ramakrishnan et al. (2012) |
| GR224 | GR219 (pGIR459); vector integrant | Ramakrishnan et al. (2012) |
| GR225 | GR219 (pGIR479); fupA/B plasmid integrant | Ramakrishnan et al. (2012) |
| GR227 | GR219 (pGIR474); fslESchu plasmid integrant | Ramakrishnan et al. (2012) |
| GR228 | GR219 (pGIR477); fupA plasmid integrant | Ramakrishnan et al. (2012) |
Work with LVS strains was conducted in a Biosafety level 2 laboratory while work with Schu S4 derivatives and with LVS strains complemented with Schu S4-derived genes was conducted in accordance with Select Agent Regulations of the US Centers for Disease Control in an approved Biosafety level 3 facility.
Complementation of ΔfupA/B and ΔfslE ΔfupA/B strains.
Use of a suicide plasmid targeted to the 3′ region of feoB for complementation with fupA, fupA/B and fslESchu in cis has been previously described (Sen et al., 2010; Ramakrishnan et al., 2012). The fslE gene from the LVS genome (fslELVS) was amplified and cloned in plasmid pSC1 as detailed previously for the fslE from Schu S4 (fslESchu) (Sen et al., 2010). For construction of the fupA+fupB complementing plasmid pBAS3, the fupA+fupB sequences from Schu S4 genomic DNA were amplified with primers 5′-CTACTGTCCGGATTTGGTTTGCCAATTTTTTATTAG and 5′-CTACTGGCGGCCGCCTAAATATGTCTAGATATAAACTG (letters in italics indicate sequences not present in the F. tularensis genome) using FastStart High Fidelity polymerase (Roche) and cloned as an NheI–NotI fragment in the suicide vector. Expression of the complementing genes on the integrative plasmids was under control of the fslA promoter. Integrants were confirmed by PCR analysis of genomic DNA from kanamycin-resistant transformants.
Growth and siderophore utilization.
Growth of F. tularensis on iron-limiting agar was essentially as previously described (Sen et al., 2010). Bacteria grown in CDM were washed and resuspended in che-CDM to an OD600 of 1.0. The cells were tenfold serially diluted in che-CDM and 5 µl of the dilutions was spotted on a CDM-Fe plate and, in parallel, on an iron-rich MHA plate. Growth at 37 °C was monitored over a period of 4 days. Cells were tested for siderophore utilization as described previously (Sullivan et al., 2006). Cells grown in CDM were washed and ~3×105 c.f.u. (10−3 dilution of cells at an OD600 of 1.0) were spread on the CDM-Fe plates and allowed to dry. Then, 2.5 µl of the siderophore-producing control strain at an OD600 of 1.0 was spotted at the centre of the seeded plates and the plates were incubated at 37 °C for 2–4 days. A growth halo of colonies around a spot demonstrated the ability of the indicator strain (seeded on the plate) to grow on the iron-limiting plate utilizing the siderophore secreted by the donor strain (spotted in the centre of the plate). Experiments were repeated on at least two different occasions with similar results.
55Fe uptake assays.
55FeCl3 (25.04 and 36.88 mCi ml−1; Perkin-Elmer) was used in transport assays carried out in 0.2 µm 96-well filter plates (Millipore) as previously described (Ramakrishnan et al., 2012). Bacteria were cultured for 20–24 h in iron-limiting che-CDM; for assessing transport rates of LVS over a range of ferrous iron concentrations, the bacteria were more completely iron-starved by further dilution and growth in iron-limiting medium for an additional16 h. Bacteria were washed to remove any iron and secreted siderophore and resuspended in che-CDM without iron to an OD600 of 0.2. Bacteria were incubated in wells of the filter plate on a heat block at 37 °C and uptake reactions were initiated by addition of 55Fe labelling mix. Bacteria were collected and washed by vacuum filtration. All experiments used samples in triplicate or quadruplicate. Individual wells were punched out and counted in scintillation fluid (Ecoscint A; National Diagnostics). Filter wells with unlabelled bacteria were used in a bicinchoninic acid assay (Pierce) to normalize results to protein. Ferrous iron-uptake reactions contained 5 mM ascorbate to keep the iron in the reduced form. Siderophore-mediated ferric iron uptake was carried out in che-CDM containing 10 mM sodium citrate. Siderophore purified from LVS culture supernatants (Sullivan et al., 2006) was incubated with 55FeCl3 in a 3 : 1 molar ratio to form complexes prior to the uptake assay and used at a concentration of 1.5 µM 55Fe and 5 µM siderophore in the uptake reaction. Incorporation of label by bacteria at two time points 5 min apart early in the uptake process was determined. The change in incorporation was normalized to time and to OD600 or to protein content to determine the rate of transport. Kinetic parameters of transport were determined by non-linear regression analysis and graphs were plotted using Prism 4.0 software (GraphPad Software). Results comparing different strains were analysed for statistical significance by Student’s t-test using Microsoft Excel.
Preparation of outer membranes.
Outer membranes from LVS and the ΔfslE and ΔfupA/B mutants were prepared following osmotic lysis using a modification of the protocol of Huntley et al. (2007). Briefly, bacteria were grown in iron-limiting che-CDM to late-exponential phase. The pelleted cells were first resuspended in 0.75 M sucrose in 10 mM Tris, pH 7.8, and subsequently adjusted to 10 mM EDTA. Lysozyme was added at 300 µg ml−1 and after 2 h of incubation on ice, the spheroplast solution was poured very slowly over four volumes of MilliQ water. After further incubation at room temperature for 30 min to effect complete cell lysis, unlysed cells were removed by centrifugation at 10 000 g for 30 min. The supernatant was centrifuged at 200 000 g for 2 h at 4 °C in an Optimax Max-XP ultracentrifuge (Beckman-Coulter) to obtain total membrane pellets. The pellet with membrane was washed once with 10 mM HEPES, pH 6.8, and then incubated in 10 mM HEPES and 0.5 % Sarkosyl at room temperature for 2 h to solubilize the inner membrane. The outer membrane fraction was obtained by centrifugation at 200 000 g for 2 h at 4 °C. The outer membrane fraction was resuspended in 100 µl of 0.1 % SDS and the concentration of proteins present was assessed using a bicinchoninic acid assay kit (Pierce).
Western blotting.
Bacteria grown in iron-replete or iron-limiting media were normalized to cell density and lysates corresponding to ~3×108 c.f.u. were separated by SDS-PAGE on 10 % gels. Outer membrane proteins in amounts as specified were also analysed on gels. Following transfer to PVDF membranes and incubation with primary and horseradish-conjugated secondary antibodies, proteins of interest were detected by chemi-luminescence. Rabbit antiserum raised to an FslE peptide (Sen et al., 2010) was used at a 1 : 2500 dilution and guinea pig antibody to the FupA amino-terminal domain (Ramakrishnan et al., 2012) was used at 1 : 5000 dilution. Rabbit antibody to FipB (Qin et al., 2011), a kind gift from Dr Barbara Mann (University of Virginia), was used at a dilution of 1 : 25 000.
Results
Ferrous iron uptake in LVS and role of FupA/B
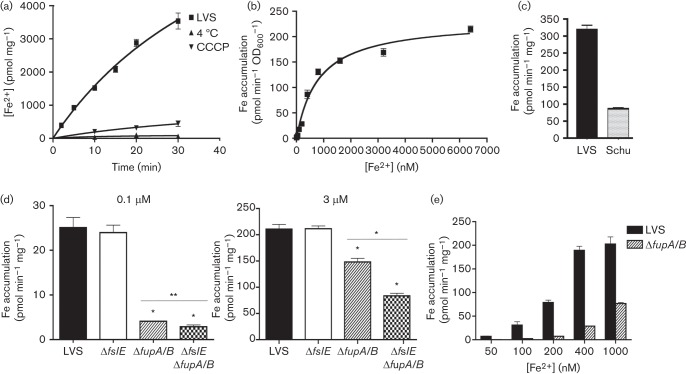
We first assessed ferrous iron uptake in LVS after growth in iron-limiting media to fully derepress iron-acquisition mechanisms, as done previously for the virulent Schu S4 (Ramakrishnan et al., 2012). We followed transport of 55Fe in the presence of ascorbate to keep the iron in the reduced form and found that 55Fe incorporation increased with time at 37 °C (Fig. 1a). This accumulation was inhibited at 4 °C and by the protonophore carbonyl cyanide m-chlorophenyl hydrazone, indicating a dependence on temperature and the proton motive force (Fig. 1a). We assessed rates of uptake over a range of ferrous iron concentrations from 6.2 nM to 6.4 µM (Fig. 1b). Based on our understanding of bacterial growth, transport at the low end of the concentration range would depend on mechanisms active under conditions of iron limitation (high-affinity uptake systems) (Sullivan et al., 2006). The transport rates were plotted as a function of iron concentration and fitted a curve representative of Michaelis–Menten kinetics, with a maximal uptake value (Vs) of 232.5 pmol min−1 OD600−1 and substrate concentration for half-maximal transport (Ks) of 815 nM (Fig. 1b). While generally resembling transport in Schu S4, the high Vmax and Ks for LVS compared with the corresponding values for Schu S4 (21.94 pmol min−1 OD600−1 and 357 nM, respectively) (Ramakrishnan et al., 2012) suggested that LVS possessed an intrinsically higher capacity for ferrous iron transport when iron was abundant. This was confirmed in a direct comparison of transport rates of the two strains with 3 µM ferrous iron, with LVS showing an ~4.5-fold higher rate of transport than Schu S4 (Fig. 1c). This difference between the two strains could either result from a conserved mechanism that was more active in LVS or be due to occurrence of different mechanisms for ferrous iron acquisition.
Fig. 1.
Ferrous iron uptake in LVS and mutants. (a) Kinetics of 55Fe2+ transport by LVS. LVS bacteria were grown in iron-limiting che-CDM for 16 h and then diluted and further grown under iron limitation for 22 h. The bacteria were washed and incubated with 7.4 µM 55Fe2+ at 37 °C and incorporation of 55Fe2+ over time was determined by scintillation counting. Transport reactions were carried out in parallel at 4 °C and with bacteria that had been pretreated with carbonyl cyanide m-chlorophenyl hydrazone. (b) Rate of 55Fe2+ transport by LVS plotted as a function of ferrous iron concentration in the uptake reaction. (c) Rate of 55Fe2+ transport by LVS and Schu S4 at 3 micromol ferrous iron. (d) Rates of high-affinity (0.1 µM) and low-affinity (3 µM) ferrous iron transport in LVS and the ΔfslE and ΔfupA/B mutants after growth in iron-limiting media for 20 h. (e) Comparison of ferrous iron transport rates of LVS and ΔfupA/B mutant over a range of iron concentrations. 55Fe accumulation was normalized to protein content or to cell density (OD600). Values are plotted as means with se. Significance was calculated relative to LVS values: *P<0.001, **P<0.02.
We compared rates of 55Fe2+ transport in LVS and ΔfslE, ΔfupA/B and the ΔfslE ΔfupA/B mutant strains at 0.1 µM Fe2+ reflecting high-affinity transport, and at 3 µM Fe2+ corresponding to a low-affinity process (Fig. 1d). The ΔfslE strain showed transport similar to LVS at both the limiting and the high iron concentrations but the ΔfupA/B and the double mutant were defective in uptake at both concentrations. We compared LVS and the ΔfupA/B strain at ferrous iron concentrations ranging from 50 nM to 1 µM (Fig. 1e). The fupA/B mutant was more defective at lower concentrations; at 50 nM there was a 20-fold difference in transport rates, while at 1 µM the difference was only 2.5-fold. These results indicated that FupA/B functioned in high-affinity ferrous iron transport, similar to FupA in Schu S4.
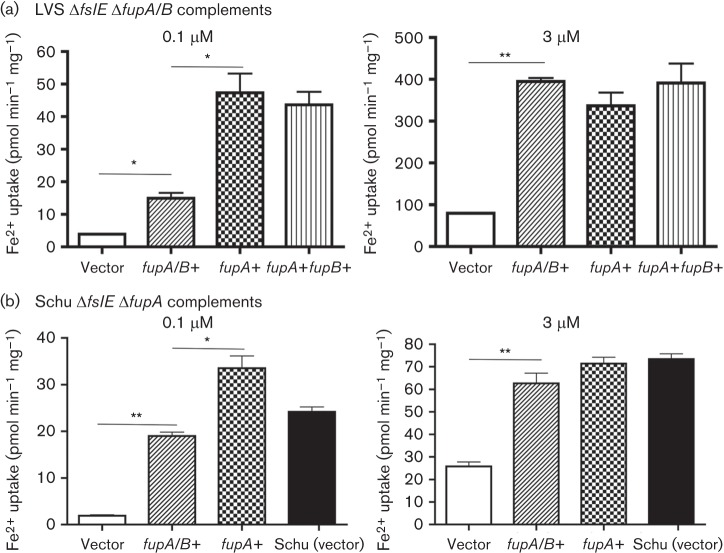
To understand how FupA/B may differ in function from FupA, we compared ferrous iron transport rates of the LVS ΔfslE ΔfupA/B mutant complemented in cis with either fupA/B or with fupA derived from the Schu S4 genome. As seen in Fig. 2(a), the low level of transport at 0.1 µM Fe2+ by the mutant transformed with vector alone was increased sixfold when fupA/B was reintroduced into the strain. Introduction of fupA in place of fupA/B resulted in a further ~150 % increase in transport rate. At 3 µM Fe2+, fupA/B and fupA appeared to have equivalent activity. Introduction of fupB along with fupA did not significantly alter iron transport in the LVS background. This is consistent with our previous findings that fupB is not critically important for iron transport in Schu S4 (Ramakrishnan et al., 2012).
Fig. 2.
Ferrous iron uptake in fupA/B and fupA complemented strains. Rates of high-affinity (0.1 µM) and low-affinity (3 µM) ferrous iron transport in (a) LVS ΔfslE ΔfupA/B and (b) Schu ΔfslE ΔfupA complemented in cis with fupA/B, fupA and fupB as indicated. Vector indicates control strains in which the vector plasmid alone was integrated in the chromosome. Values are plotted as means with se; *P<0.01; **P<0.001.
To test if transport by FupA and FupA/B might be influenced by the strain background, we performed the complementary experiment, introducing fupA/B and fupA individually into the Schu S4 ΔfslE ΔfupA strain. Wd found that fupA/B was able to promote ferrous iron transport in the Schu S4 background, but as seen with the LVS strain, the transport rate at 0.1 µM Fe2+ was lower than that conferred by fupA (Fig. 2b) . At 3 µM iron, there was no difference in the rates of transport. Thus, fupA/B appeared to function less efficiently than fupA in high-affinity ferrous iron transport regardless of the genetic background of the host strain.
To confirm that the transport differences with the fupA/B and fupA complements were not caused by differences in expression of these genes, we tested lysates from the different strains in Western blotting with antibody raised to the amino-terminal domain of FupA that is also present in FupA/B. In this complementation system, expression of the gene of interest is under control of the Fur-regulated fslA promoter and is enhanced relative to the wild-type strain, although it is still influenced by iron levels (Ramakrishnan et al., 2012). We determined that the complementing gene products were expressed at comparable levels in the different transformants of LVS ΔfslE ΔfupA/B (Fig. 3a) and Schu S4 ΔfslE ΔfupA (Fig. 3b). We also assessed levels of the FupA and FupA/B proteins in the outer membranes of the Schu S4 ΔfslE ΔfupA complements. As a loading control, we used antibody to FipB (FTL_1096 in LVS, orthologue of FTT1103 in Schu S4) (Qin et al., 2011), a lipoprotein that fractionates with the outer membrane (Huntley et al., 2007). Both FupA and FupA/B were present at comparable levels in the outer membrane, implying that the FupA/B fusion protein is intrinsically less efficient at ferrous iron transport than FupA.
Fig. 3.
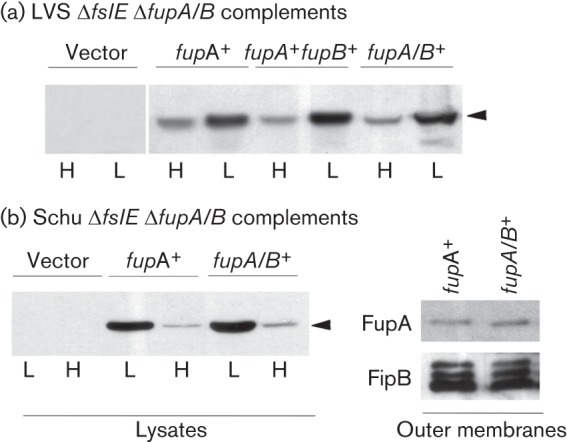
Expression of fupA and fupA/B in complemented strains. Lysates of bacteria grown in iron-replete (H) or iron-limiting (L) media were analysed by SDS-PAGE and Western blotting with antibodies to FupA (FupA/B) and control FipB as indicated. The arrowhead indicates the FupA band. Vector indicates strains carrying the control vector plasmid. (a) Whole-cell lysates of LVS ΔfslE ΔfupA/B complemented with different genes as indicated were tested for expression of FupA (FupA/B). (b) Whole-cell lysates of Schu ΔfslE ΔfupA complemented with fupA and fupA/B mutants were tested for levels of FupA (FupA/B). Corresponding outer membrane proteins (1 µg) were analysed by Western blotting for levels of FupA (FupA/B) and for control protein FipB.
Low-affinity ferrous iron transport (at 3 µM Fe2+, Fig. 2) remained distinctly different between the LVS- and Schu S4-derived strains, with the rate in all the LVS-based complements being about fivefold higher than in the Schu S4 background (~300 versus ~60 pmol min−1 mg−1). This indicated that the capacity for ferrous iron uptake under iron-replete conditions was not influenced by FupA or FupA/B, but was instead set by some additional mechanism that differs between the LVS and Schu S4 backgrounds.
FupA/B is more effective than FupA at supporting growth on iron-limiting agar
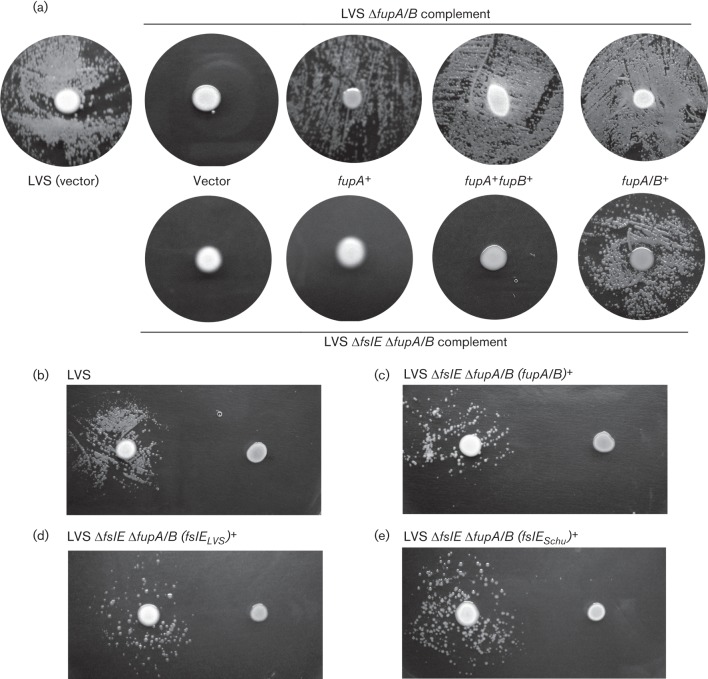
A ΔfupA/B mutant of LVS shows reduced growth on iron-limiting agar while deletion of both fupA/B and fslE results in a more severe growth defect (Sen et al., 2010). We compared the ability of fupA and fupA/B introduced in cis to support growth of LVS ΔfupA/B and LVS ΔfslE ΔfupA/B under iron limitation. Serial dilutions of washed bacteria were spotted on CDM-Fe agar and growth was compared with that on iron-replete MHA (Fig. 4). While the LVS vector control grew out to four dilutions on the iron-limiting agar, the ΔfupA/B vector control grew only to the second dilution and the double mutant control showed even poorer growth. As seen previously (Sen et al., 2010), complementation with fupA/B restored growth similarly in both strains, indicating that overexpression of FupA/B can compensate in some way for loss of FslE function. Interestingly, the fupA and the fupA+fupB complements showed differences in growth recovery in the two mutant backgrounds. The single mutant complemented with fupA or with fupA+fupB grew similarly to the fupA/B complement; in contrast, the fupA and fupA+fupB complemented double mutant displayed poor growth that was only marginally better than the vector control. These results demonstrated that fupA could compensate for loss of fupA/B only in conjunction with fslE. Additional loss of fslE gene function could be compensated for by restoration of fupA/B expression, but not of fupA.
Fig. 4.
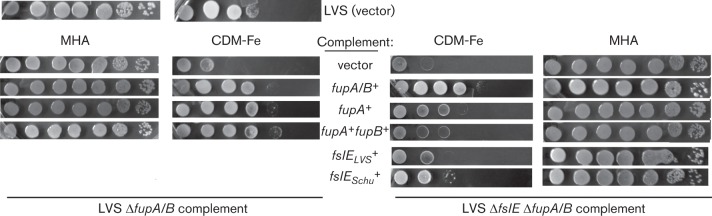
Growth of LVS ΔfupA/B and LVS ΔfslE ΔfupA/B complements on iron-limiting agar. Tenfold serial dilutions of LVS ΔfupA/B or LVS ΔfslE ΔfupA/B complemented with different genes as indicated were spotted on iron-replete (MHA) or iron-limiting (CDM-Fe) plates. LVS carrying the vector alone was used as control. Growth was recorded after 2 days (MHA) or 4 days (CDM-Fe).
To determine if the growth promotion by fupA/B is specific to the LVS background, we similarly tested iron-limited growth of the fupA/B complemented Schu ΔfslE ΔfupA mutant (Fig. 5). As previously shown, restoration of the Schu S4-derived fslE (fslES) in cis to this mutant can partially rescue growth, whereas restoration of fupA cannot (Ramakrishnan et al., 2012). Interestingly, the introduction of fupA/B resulted in growth that was better than the fslE complement and almost comparable to the parent Schu S4 vector control. This indicated that FupA/B but not FupA possessed a strain-independent ability to promote growth under iron limitation and could compensate for loss of fupA and of fslE.
Fig. 5.
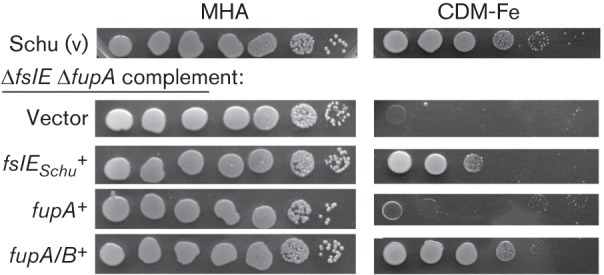
Growth of Schu ΔfslE ΔfupA complements on iron-limiting agar. Serial dilutions of Schu ΔfslE ΔfupA complemented with endogenous fslE (fslESchu), fupA or fupA/B as indicated were tested for growth on iron-replete (MHA) or on iron-limiting (CDM-Fe) agar as in Fig. 4. Growth of the control Schu S4 bearing the vector alone is shown.
FupA/B but not FupA promotes siderophore utilization
Siderophore-promoted growth assays on iron-limiting agar using different mutant strains showed that FslE was essential for siderophore utilization in Schu S4 and in the related Francisella novicida strain U112 (Kiss et al., 2008; Ramakrishnan et al., 2008). In LVS, however, fupA/B and not fslE was primarily required for growth in this cross-feeding assay (Sen et al., 2010).
To better understand the basis for differences in growth promotion by FupA, FupA/B and FslE, we tested the different complemented strains for the ability to utilize siderophore for growth (Fig. 6a). The test bacteria were spread on iron-limiting agar and cells of siderophore-producing LVS were spotted in the centre. The siderophore secreted from the bacteria in the centre could promote growth of the surrounding LVS control bacteria. The single and double mutants bearing the vector control were unable to produce growth haloes around the siderophore-producing strain, as shown previously (Sen et al., 2010). We found that introduction of fupA or fupA+fupB in the single mutant promoted robust growth, similar to restoration of fupA/B expression. The ΔfslE ΔfupA/B mutant, however, was only able to grow upon restoration of fupA/B expression, but not by introduction of fupA or fupA+fupB. That the growth halo is siderophore-dependent may be confirmed by the inability of a siderophore-deficient ΔfslA strain to promote this growth; thus, LVS seeded on the iron-limiting agar can form a halo around an LVS spot but not around a spot of ΔfslA (Sullivan et al., 2006) (Fig. 6b). Likewise, the LVS ΔfslE ΔfupA/B mutant complemented with fupA/B was only able to grow around the LVS spot but not the ΔfslA cells (Fig. 6c). Our results thus indicated that fupA/B could mediate siderophore utilization whereas fupA was unable to facilitate siderophore-mediated iron acquisition on its own, although it could do so in conjunction with fslELVS. We also found that fupA/B was similarly functional in siderophore utilization in the Schu S4 background (data not shown).
Fig. 6.
Growth promotion by cross-feeding of siderophore. (a) Growth promotion of complements by LVS: 3×105 c.f.u. of bacterial strains as indicated were seeded on iron-limiting CDM agar and siderophore-producing LVS bacteria carrying the vector control were spotted in the centre. Growth halo formation around the spot was recorded after 3 days at 37 °C. (b–e) Siderophore dependence of growth halo formation. Bacteria as indicated were seeded on iron-limiting agar and tested for the ability to form growth haloes around spots of siderophore- producing LVS (left) and siderophore-deficient ΔfslA bacteria (right) individually spotted on the same plates. The strains were seeded at titres as follows: (b) 3×105 c.f.u. LVS; (c) 3×106 c.f.u. LVS ΔfslE ΔfupA/B complemented with fupA/B; (d) 3×107 c.f.u. LVS ΔfslE ΔfupA/B complemented with fslELVS; (e) 3×106 c.f.u. LVS ΔfslE ΔfupA/B complemented with fslESchu.
FslELVS is less effective than FslESchu at mediating siderophore utilization for growth under iron limitation
The importance of FupA/B for siderophore-dependent growth and the apparent minor role for FslELVS is in contrast to the situation in Schu S4, where FslE is primarily responsible for the process (Sen et al., 2010; Ramakrishnan et al., 2012). The FslE sequence in LVS (FslELVS) differs at five amino-acid residues from that of Schu S4 (FslESchu) and we considered the possibility that the mutations may either destabilize the FslELVS protein, leading to reduced levels in the outer membrane, or might reduce the functionality of the protein. To test the first possibility, we used Western blotting to examine levels of the proteins in lysates and in the outer membrane of LVS and the single mutant strains (Fig. 7a). FipB was used as loading control. FupA/B was present at similar levels in lysates and in membrane fractions of LVS and the ΔfslE mutant, while no band corresponding to FupA/B was detected in the ΔfupA/B mutant. FslE was detectable in the lysate and also in the outer membrane fraction of LVS. The fsl operon is deregulated in the ΔfupA/B mutant (Sen et al., 2010); as expected, levels of FslE were much greater in the ΔfupA/B mutant lysate and this deregulation was observed also in the outer membrane protein fraction (Fig. 7a). The results demonstrated that FslELVS does localize to the outer membrane and that its assembly is not dependent on FupA/B.
Fig. 7.
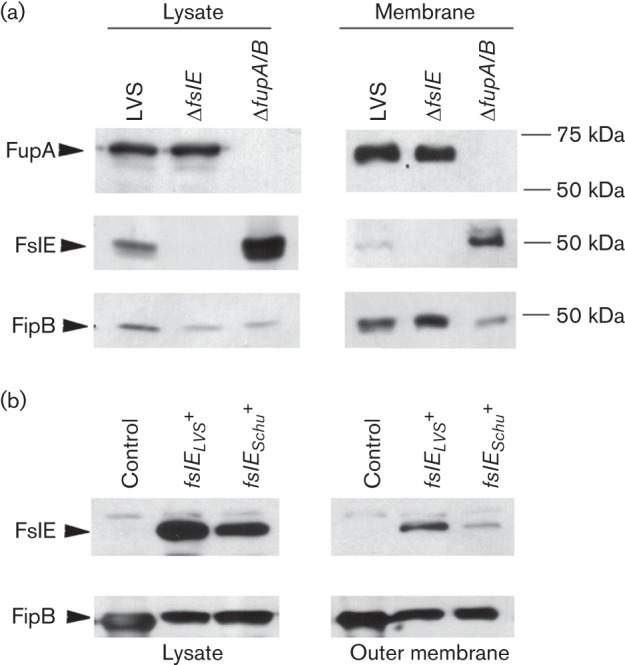
Expression of fslE in bacterial strains. Whole-cell lysates and outer membrane preparations from bacteria as indicated were analysed by Western blotting for expression of FslE and FupA/B. FipB was used as loading control. (a) Lysates of LVS and ΔfslE and ΔfupA/B mutants and corresponding outer membrane proteins (5 µg) were analysed. (b) Lysates of LVS ΔfslE ΔfupA/B complemented with fslE derived from LVS (fslELVS) or from Schu S4 (fslESchu) and corresponding outer membrane proteins (2 µg) were analysed.
We introduced the fslE orthologues independently into the LVS ΔfslE ΔfupA/B strain and tested the levels of these proteins in lysates and in the outer membrane by Western blotting, with FipB as loading control (Fig. 7b). FslELVS in lysates and in the membrane fraction was detected at levels not lower but higher than FslESchu. These results suggested that the sequence alterations in FslELVS do not destabilize the protein. To test if the sequence differences might lead to reduced functionality of FslELVS, we compared growth of the complements on iron-limiting CDM agar. As shown in Fig. 4, there was an obvious difference in the restoration of growth by the two orthologues. The fslESchu complement showed more robust growth than the fslELVS complement, suggesting that FslELVS is indeed less effective than FslESchu at promoting growth under iron limitation.
We tested the LVS ΔfslE ΔfupA/B strain complemented with each of the fslE orthologues in the siderophore utilization assay on iron-limiting CDM agar. As shown in Fig. 6(d, e), both complements formed growth haloes around sideropore-producing LVS but not around the siderophore-deficient ΔfslA mutant spots. Of note, the ability to form growth haloes was dependent on the number of c.f.u. seeded on the iron-limiting plate; while ~3×105 c.f.u. of LVS seeded on the plate were sufficient for growth halo formation, the fslESchu complement of LVS ΔfslE ΔfupA/B required ~3×106 c.f.u. and the fslELVS complement required ~3×107 c.f.u. These results are consistent with differences in growth of serial dilutions of the strains (Fig. 4).
FupA/B and FslELVS jointly mediate siderophore–55Fe transport
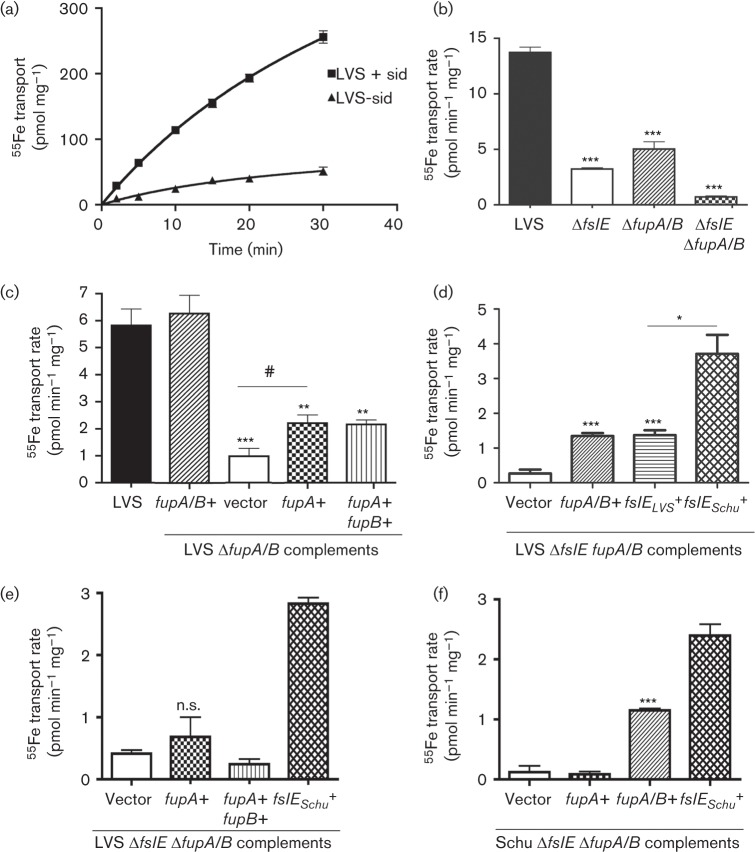
Although growth of Schu S4 on iron-limiting CDM agar primarily requires FslE function, optimal growth relies on both FslE and FupA, indicating that the trace iron in the medium is acquired both in the ferrous form and through siderophore–ferric iron complexes (Ramakrishnan et al., 2012). The most direct read-out for siderophore-mediated iron acquisition is bacterial incorporation of 55Fe complexed to siderophore in real-time and we have previously used this assay to establish the role of FslE as siderophore transporter in Schu S4 (Ramakrishnan et al., 2012). We characterized siderophore-dependent ferric iron uptake in LVS so that we could more definitively understand the roles of fupA/B and fslELVS in this process. LVS bacteria grown for an extended period under iron limitation were incubated with 55Fe complexed to purified siderophore in the presence of citrate and accumulation of 55Fe was followed over time. The bacteria showed a siderophore-dependent increase in internalized 55Fe with time (Fig. 8a). A slow increase in the absence of added siderophore was probably due to endogenous siderophore produced by the bacteria.
Fig. 8.
Siderophore-mediated 55Fe transport. (a) Siderophore-dependent accumulation of 55Fe by LVS. LVS bacteria were grown for 16 h under iron limitation, and diluted and further grown for 24 h in iron-limiting media. The bacteria were washed and incubated with 55Fe in the presence (+sid) or absence (-sid) of siderophore and incorporation of 55Fe over time was determined by scintillation counting. (b) Siderophore-mediated 55Fe transport rates in LVS and ΔfslE, ΔfupA/B or ΔfslE ΔfupA/B mutants grown in iron-limiting media for 20 h. (c) Rates of siderophore-mediated 55Fe transport in LVS ΔfupA/B complemented with different genes as indicated. (d) and (e) Rates of siderophore-mediated 55Fe transport in LVS ΔfslE ΔfupA/B complemented by fupA/B and by fslE orthologues from the LVS and Schu S4 backgrounds. (f) Rates of siderophore-mediated 55Fe transport in Schu ΔfslE ΔfupA/B complemented with different genes as indicated. 55Fe accumulation was normalized to protein content. Values are plotted as means with se. Significance was calculated relative to LVS values in (b) and (c) and relative to vector control in (d), (e) and (f): #P<0.05, *P<0.01, **P<0.002, ***P<0.001; n.s., not significant.
We compared rates of siderophore-mediated 55Fe transport in LVS and the fslE and fupA/B mutants (Fig. 8b). Somewhat surprisingly, both of the single mutants showed marked reduction in transport rates, suggesting that both FslE and FupA/B individually contribute to siderophore-mediated acquisition of iron in LVS. The double mutant showed no appreciable transport of 55Fe, suggesting that these two proteins are the sole mediators of siderophore–iron transport in LVS.
To further analyse the roles of fupA/B and fslE in siderophore–iron transport, we examined the rates of siderophore-mediated 55Fe transport in ΔfupA/B mutants complemented with the different paralogues (Fig. 8c). The ΔfupA/B mutant with control vector had a low rate of transport compared with LVS. The rate was restored to wild-type levels when fupA/B was provided in cis. Introduction of fupA or fupA+fupB resulted in a twofold enhancement of uptake over the vector control, but not to wild-type levels. We examined transport in the double mutant complemented with either fupA/B or fslELVS in cis. Restitution of fupA/B and fslELVS individually partially restored transport, but neither alone restored activity to wild-type levels (Fig. 8d), demonstrating that each of the paralogues contributes to siderophore-mediated iron acquisition. Transport in the fslEschu complemented double mutant was notably increased over the fslELVS complement, demonstrating that FslELVS is less effective at siderophore–iron acquisition than FslESchu. Neither the fupA nor the fupA+fupB complement showed increased siderophore–iron transport over the vector control (Fig. 8e). We also examined siderophore-mediated iron transport in the Schu ΔfslE ΔfupA mutant complemented with the different paralogues. The fupA/B but not the fupA complement showed an increase in siderophore-mediated ferric iron transport, as in the LVS background (Fig. 8f).
Overall, these experiments demonstrated that both FupA/B and FslELVS independently contribute to siderophore-mediated iron acquisition. Comparison of transport by the single mutants with LVS in Fig. 8(b) suggests, however, that the paralogues may function not additively but co-operatively in transport. Although FupA did not have siderophore-transport activity, its ability to increase transport activity in the ΔfupA/B mutant (Fig. 8c) suggested that FupA could support FslELVS function in this process in some manner.
Discussion
Specialized mechanisms for acquiring iron are critical for survival under iron limitation and bacterial pathogens typically possess multiple redundant systems for obtaining this nutrient within the host milieu (Ratledge & Dover, 2000). The small 1.892 Mb genome of Schu S4 (Larsson et al., 2005) encodes only two transporters for moving iron across the outer membrane under limiting conditions: FslE is a siderophore–ferric iron transporter, while the paralogous protein FupA mediates high-affinity ferrous iron transport (Ramakrishnan et al., 2012). The LVS genome shares 99.3 % identity with that of Schu S4 and one of the earliest noted genomic differences between the strains (RD18) was the deletion in the fupA–fupB region leading to formation of the fupA/B hybrid gene in LVS (Broekhuijsen et al., 2003; Rohmer et al., 2006; Svensson et al., 2005). The switch from FupA to FupA/B in LVS profoundly influences virulence (Salomonsson et al., 2009), suggesting that this altered protein is altered in function as well. Here we have shown that the FupA/B protein of LVS retains high-affinity ferrous iron transport ability, albeit at a somewhat diminished level compared with FupA of Schu S4. We found additionally that FupA/B is capable of siderophore–ferric iron transport in conjunction with FslELVS while FslELVS itself has reduced functionality in siderophore–iron transport as compared with FslESchu. These differences may render LVS less efficient at extracting nutrient iron within the limiting host environment.
Strains of F. tularensis subsp. tularensis and subsp. holarctica show differences in iron metabolism, as evidenced by the larger internal iron stores of subsp. holarctica strains following growth under standard iron-replete conditions (Hubálek et al., 2004; Lindgren et al., 2011). A greater susceptibility to oxidative stress accompanies the increased iron stores in the F. tularensis subsp. holarctica strains and was suggested as contributing to the reduced virulence of these strains (Lindgren et al., 2011). An inherent difference in capacity for ferrous iron uptake may contribute to the altered iron stores; we have shown here that under iron-replete conditions, LVS has a four- to fivefold greater rate of ferrous iron uptake than Schu S4. This increased rate of transport is not determined by the presence of FupA/B in the outer membrane, but by some other aspect of metabolism.
Iron-acquisition mechanisms are mechanistically conserved in Gram-negative bacteria. In the overwhelming majority of Gram-negative bacteria, transport of the siderophore–iron complex across the outer membrane is an energy-requiring process and is enabled by the TonB–ExbB–ExbD complex, which transduces energy from the proton motive force (Faraldo-Gómez & Sansom, 2003). The Francisella genome does not encode proteins of the TonB complex and iron–siderophore transport by FslE represents a novel paradigm (Ramakrishnan et al., 2008). A TonB-independent siderophore transporter that may be mechanistically similar to FslE has also been identified in Legionella species (Chatfield et al., 2011). FslE and the FupA and FupA/B paralogues are predicted by the PRED-TMBB program (Bagos et al., 2004a, b) to assemble in the outer membrane as beta-barrels with extracellular loops and amino-terminal periplasmic extensions, resembling the plug and barrel structure of classic TonB-dependent transporters (Noinaj et al., 2010). While the proton motive force is important for siderophore–iron transport in F. tularensis (Ramakrishnan et al., 2012, and data not shown), it remains to be determined whether an energy-transduction mechanism analogous to TonB is associated with transport across the outer membrane.
We have demonstrated here that although siderophore–55Fe transport in LVS displays similarity to the process in Schu S4, FslELVS is functionally less efficient than FslESchu. FslELVS and FslESchu display differences in five amino-acid residues across their length of 509 aa. Two of the sequence alterations in FslELVS compared with FslESchu are conservative substitutions (V154I and M169I) mapping to the predicted plug domain, while the three other changes (T216A, K439E, S472A) map to the predicted barrel domain. Substrate specificity in the TonB-dependent transporters is conferred by sequences of the barrel domain, extracellular loops and the periplasmic plug (Noinaj et al., 2010). The sequence changes in FslELVS could conceivably impact siderophore–iron transport function of the protein by altering affinity for substrate. Interestingly, these sequence changes are conserved in the different sequenced strains of F. tularensis subsp. holarctica in the GenBank database and may represent an evolutionary characteristic of this subspecies.
Previous studies indicated that while FslE functions in siderophore–iron transport in Schu S4, the contribution of FslE to iron acquisition is secondary to FupA/B in LVS (Ramakrishnan et al., 2008, 2012; Sen et al., 2010). Optimal growth on iron-limiting CDM agar requires both ferrous and ferric iron-uptake capability, and the dual involvement of FupA/B in ferrous iron uptake and in siderophore–iron transport coupled with the weaker transport activity of FslELVS could account for the dominant role played by FupA/B in growth-based assays with LVS. The 55Fe transport assays have clarified roles for the two paralogues in LVS.
FupA (557 aa) shares 63 % identity and 79 % similarity overall with FslE and has high specificity for ferrous iron but no appreciable siderophore–iron transport capability. FupB (481 aa), a paralogue with 50 % identity and 66 % similarity to FupA and with lower sequence similarity to FslE (42 % identity, 59 % similarity) is also not associated with transport of either form of iron. FupA/B largely retains the ferrous iron transport capability of FupA while having acquired some level of siderophore-iron transport capability. While both FslELVS and FupA/B have siderophore–ferric iron transport capability, our 55Fe transport studies suggest that these paralogues may function co-operatively in transport. How FupA/B co-operates with FslELVS to carry out siderophore-mediated iron uptake remains to be discerned in future studies. Neither FupA nor FupA in combination with FupB is capable of promoting siderophore transport, but we found that they could enhance FslELVS transport function in some manner that may be related to a co-operative interaction. Identification of the residues in the predicted plug and barrel domains that contribute to altered substrate specificity could provide insight into the transport mechanism. Ferrous iron transport in Schu S4 and LVS is also dependent on the proton motive force, and given the homology between FupA and FupA/B with FslE, a potential involvement of an energy-transduction mechanism in the transport of ferrous iron across the outer membrane is also a question to investigate.
The fupA/B recombination event in LVS is associated with attenuation in virulence by the intradermal or subcutaneous route, although the strain remains highly virulent if inoculated intraperitoneally in mice (Fortier et al., 1991; Salomonsson et al., 2009). However, deletion of the fupA/B gene results in significant attenuation also by the intraperitoneal route of infection and deletion of the fslE locus in addition leads to further attenuation (Sen et al., 2010). FupA/B and FslE proteins appear to be the only significant outer membrane transporters in LVS that operate under iron limitation, consistent with the roles of FupA and FslE in Schu S4. The accumulated evidence suggests that the iron-acquisition functions of these paralogues are critical for survival of F. tularensis as pathogens in the mammalian host.
Acknowledgements
We thank Suzi Chung for help with cloning the fslELVS gene and Natalie Perez for helpful discussions. We thank Dr Barbara Mann and Dr Aiping Qin for antibody to FipB. This work was supported by a National Institutes of Health grant (AI067823) to G. R. and by intramural funding from the University of Virginia School of Medicine.
Abbreviations:
- LVS
live vaccine strain
References
- Bagos P. G., Liakopoulos T. D., Spyropoulos I. C., Hamodrakas S. J. (2004a). PRED-TMBB: a web server for predicting the topology of beta-barrel outer membrane proteins. Nucleic Acids Res 32 (Web Server issue), W400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagos P. G., Liakopoulos T. D., Spyropoulos I. C., Hamodrakas S. J. (2004b). A Hidden Markov Model method, capable of predicting and discriminating beta-barrel outer membrane proteins. BMC Bioinformatics 5, 29. 10.1186/1471-2105-5-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekhuijsen M., Larsson P., Johansson A., Byström M., Eriksson U., Larsson E., Prior R. G., Sjöstedt A., Titball R. W., Forsman M. (2003). Genome-wide DNA microarray analysis of Francisella tularensis strains demonstrates extensive genetic conservation within the species but identifies regions that are unique to the highly virulent F. tularensis subsp. tularensis. J Clin Microbiol 41, 2924–2931. 10.1128/JCM.41.7.2924-2931.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D. S. (1977). Immunization against tularemia: analysis of the effectiveness of live Francisella tularensis vaccine in prevention of laboratory-acquired tularemia. J Infect Dis 135, 55–60. 10.1093/infdis/135.1.55 [DOI] [PubMed] [Google Scholar]
- Chamberlain R. E. (1965). Evaluation of live tularemia vaccine prepared in a chemically defined medium. Appl Microbiol 13, 232–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield C. H., Mulhern B. J., Burnside D. M., Cianciotto N. P. (2011). Legionella pneumophila LbtU acts as a novel, TonB-independent receptor for the legiobactin siderophore. J Bacteriol 193, 1563–1575. 10.1128/JB.01111-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng K., Blick R. J., Liu W., Hansen E. J. (2006). Identification of Francisella tularensis genes affected by iron limitation. Infect Immun 74, 4224–4236. 10.1128/IAI.01975-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraldo-Gómez J. D., Sansom M. S. (2003). Acquisition of siderophores in gram-negative bacteria. Nat Rev Mol Cell Biol 4, 105–116. 10.1038/nrm1015 [DOI] [PubMed] [Google Scholar]
- Fortier A. H., Slayter M. V., Ziemba R., Meltzer M. S., Nacy C. A. (1991). Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect Immun 59, 2922–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubálek M., Hernychová L., Brychta M., Lenco J., Zechovská J., Stulík J. (2004). Comparative proteome analysis of cellular proteins extracted from highly virulent Francisella tularensis ssp. tularensis and less virulent F. tularensis ssp. holarctica and F. tularensis ssp. mediaasiatica. Proteomics 4, 3048–3060. 10.1002/pmic.200400939 [DOI] [PubMed] [Google Scholar]
- Huntley J. F., Conley P. G., Hagman K. E., Norgard M. V. (2007). Characterization of Francisella tularensis outer membrane proteins. J Bacteriol 189, 561–574. 10.1128/JB.01505-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss K., Liu W., Huntley J. F., Norgard M. V., Hansen E. J. (2008). Characterization of fig operon mutants of Francisella novicida U112. FEMS Microbiol Lett 285, 270–277. 10.1111/j.1574-6968.2008.01237.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson P., Oyston P. C., Chain P., Chu M. C., Duffield M., Fuxelius H. H., Garcia E., Hälltorp G., Johansson D. & other authors (2005). The complete genome sequence of Francisella tularensis, the causative agent of tularemia. Nat Genet 37, 153–159. 10.1038/ng1499 [DOI] [PubMed] [Google Scholar]
- Lindgren H., Honn M., Golovlev I., Kadzhaev K., Conlan W., Sjöstedt A. (2009). The 58-kilodalton major virulence factor of Francisella tularensis is required for efficient utilization of iron. Infect Immun 77, 4429–4436. 10.1128/IAI.00702-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren H., Honn M., Salomonsson E., Kuoppa K., Forsberg A., Sjöstedt A. (2011). Iron content differs between Francisella tularensis subspecies tularensis and subspecies holarctica strains and correlates to their susceptibility to H2O2-induced killing. Infect Immun 79, 1218–1224. 10.1128/IAI.01116-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne T. S., Michell S. L., Diaper H., Wikström P., Svensson K., Oyston P. C., Titball R. W. (2007). A 55 kDa hypothetical membrane protein is an iron-regulated virulence factor of Francisella tularensis subsp. novicida U112. J Med Microbiol 56, 1268–1276. 10.1099/jmm.0.47190-0 [DOI] [PubMed] [Google Scholar]
- Noinaj N., Guillier M., Barnard T. J., Buchanan S. K. (2010). TonB-dependent transporters: regulation, structure, and function. Annu Rev Microbiol 64, 43–60. 10.1146/annurev.micro.112408.134247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin A., Scott D. W., Rabideau M. M., Moore E. A., Mann B. J. (2011). Requirement of the CXXC motif of novel Francisella infectivity potentiator protein B FipB, and FipA in virulence of F. tularensis subsp. tularensis. PLoS ONE 6, e24611. 10.1371/journal.pone.0024611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan G., Meeker A., Dragulev B. (2008). fslE is necessary for siderophore-mediated iron acquisition in Francisella tularensis Schu S4. J Bacteriol 190, 5353–5361. 10.1128/JB.00181-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan G., Sen B., Johnson R. (2012). Paralogous outer membrane proteins mediate uptake of different forms of iron and synergistically govern virulence in Francisella tularensis tularensis. J Biol Chem 287, 25191–25202. 10.1074/jbc.M112.371856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratledge C., Dover L. G. (2000). Iron metabolism in pathogenic bacteria. Annu Rev Microbiol 54, 881–941. 10.1146/annurev.micro.54.1.881 [DOI] [PubMed] [Google Scholar]
- Rohmer L., Brittnacher M., Svensson K., Buckley D., Haugen E., Zhou Y., Chang J., Levy R., Hayden H. & other authors (2006). Potential source of Francisella tularensis live vaccine strain attenuation determined by genome comparison. Infect Immun 74, 6895–6906. 10.1128/IAI.01006-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomonsson E., Kuoppa K., Forslund A. L., Zingmark C., Golovliov I., Sjöstedt A., Noppa L., Forsberg A. (2009). Reintroduction of two deleted virulence loci restores full virulence to the live vaccine strain of Francisella tularensis. Infect Immun 77, 3424–3431. 10.1128/IAI.00196-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen B., Meeker A., Ramakrishnan G. (2010). The fslE homolog, FTL_0439 (fupA/B), mediates siderophore-dependent iron uptake in Francisella tularensis LVS. Infect Immun 78, 4276–4285. 10.1128/IAI.00503-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöstedt A. (2007). Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann N Y Acad Sci 1105, 1–29. 10.1196/annals.1409.009 [DOI] [PubMed] [Google Scholar]
- Sullivan J. T., Jeffery E. F., Shannon J. D., Ramakrishnan G. (2006). Characterization of the siderophore of Francisella tularensis and role of fslA in siderophore production. J Bacteriol 188, 3785–3795. 10.1128/JB.00027-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson K., Larsson P., Johansson D., Byström M., Forsman M., Johansson A. (2005). Evolution of subspecies of Francisella tularensis. J Bacteriol 187, 3903–3908. 10.1128/JB.187.11.3903-3908.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twine S., Byström M., Chen W., Forsman M., Golovliov I., Johansson A., Kelly J., Lindgren H., Svensson K. & other authors (2005). A mutant of Francisella tularensis strain SCHU S4 lacking the ability to express a 58-kilodalton protein is attenuated for virulence and is an effective live vaccine. Infect Immun 73, 8345–8352. 10.1128/IAI.73.12.8345-8352.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]