Abstract
YadB and YadC are putative trimeric autotransporters present only in the plague bacterium Yersinia pestis and its evolutionary predecessor, Yersinia pseudotuberculosis. Previously, yadBC was found to promote invasion of epithelioid cells by Y. pestis grown at 37 °C. In this study, we found that yadBC also promotes uptake of 37 °C-grown Y. pestis by mouse monocyte/macrophage cells. We tested whether yadBC might be required for lethality of the systemic stage of plague in which the bacteria would be pre-adapted to mammalian body temperature before colonizing internal organs and found no requirement for early colonization or growth over 3 days. We tested the hypothesis that YadB and YadC function on ambient temperature-grown Y. pestis in the flea vector or soon after infection of the dermis in bubonic plague. We found that yadBC did not promote uptake by monocyte/macrophage cells if the bacteria were grown at 28 °C, nor was there a role of yadBC in colonization of fleas by Y. pestis grown at 21 °C. However, the presence of yadBC did promote recoverability of the bacteria from infected skin for 28 °C-grown Y. pestis. Furthermore, the gene for the proinflammatory chemokine CXCL1 was upregulated in expression if the infecting Y. pestis lacked yadBC but not if yadBC was present. Also, yadBC was not required for recoverability if the bacteria were grown at 37 °C. These findings imply that thermally induced virulence properties dominate over effects of yadBC during plague but that yadBC has a unique function early after transmission of Y. pestis to skin.
Introduction
Yersinia pestis is a vector-borne pathogen that causes plague in humans. This disease manifests in three forms, bubonic due to injection of the bacteria into the dermis by the flea vector, pneumonic due to inhalation of infectious aerosol, and septicaemic due to systemic dissemination within the body (Perry & Fetherston, 1997). The flea midgut and mammalian host present strikingly different environments for bacterial survival and growth, and temperature plays an important role in adapting gene expression to the two environments (Han et al., 2004; Hinnebusch, 2005; Motin et al., 2004). Thermally upregulated virulence factors are expressed at low basal levels in the flea vector at ambient temperature, whereas they become strongly expressed once within mammalian tissues. Major examples include adhesins, anti-phagocytic fibrils, a type III secretion system (T3SS) and its substrates called Yops that paralyse phagocytosis and proinflammatory cell biology, and an underacylated lipooligosaccharide that evades proinflammatory signalling through Toll-like receptor 4 (Felek et al., 2010; Du et al., 2002; Montminy et al., 2006; Viboud & Bliska, 2005). Together, these virulence factors promote bacterial replication to the overwhelming numbers needed for the infection of a flea via a few microlitres of blood. Transmission factors such as biofilm formation are strongly expressed in the flea at ambient temperature, promote colonization of the flea and function to promote survival immediately after transmission. An example of the last-named is the surface protease Pla, which is unique to Y. pestis and promotes dissemination from the site of the flea-bite (Chouikha & Hinnebusch, 2012; Sodeinde et al., 1992; Zhang et al., 2008). Pla continues to function as a virulence factor once in a mammal, its expression and activity increase (Chromy et al., 2005; McDonough & Falkow, 1989; Motin et al., 2004; Suomalainen et al., 2010), and its gene is among those most strongly expressed in the inflamed lymph nodes (buboes) that are pathognomonic of bubonic plague (Sebbane et al., 2006; Vadyvaloo et al., 2010).
This paper addresses the function of the yadBC operon, which is unique to Y. pestis and its immediate ancestor, the intestinal pathogen Yersinia pseudotuberculosis (Forman et al., 2008). yadBC is not found in any other organisms in the databases, including the more distantly related enteropathogenic Yersinia enterocolitica, and its acquisition was speculated to have a role in the more aggressively disseminative character of Y. pestis and Y. pseudotuberculosis compared with Y. enterocolitica or to represent a step in the evolution of the vector-borne transmission of Y. pestis (Forman et al., 2008).
The YadB and YadC proteins are predicted to be trimeric autotransporters with an architecture that has been well characterized in the prototype YadA of the enteropathogenic Yersinia. These proteins trimerize in the outer membrane, forming a 12-member beta-barrel membrane anchor from the C termini of the subunits, through which pass the three linker domains bearing the surface-exposed exported domains (Leo et al., 2012). The exported domains of the three subunits intertwine as a coiled-coil stalk ending in the three N-terminal head domains that typically have a virulence-related function such as adhesion. The predicted 61.6 kDa YadC has a 31.7 kDa head region, whereas the head region of the 35 kDa YadB is only 5.7 kDa in size. Neither head region has orthologues in the non-redundant databases (other than in strains of Y. pestis and in Y. pseudotuberculosis). In addition to the curious truncated head domain of YadB, YadB and YadC exhibit other features that are unusual for trimeric autotransporters. Most trimeric autotransporters are abundantly expressed and function as adhesins; however, the yadBC promoter was not strongly expressed, and YadB and YadC were difficult to detect even in immunoblots (Forman et al., 2008). yadBC also did not promote adherence of Y. pestis to epithelioid cells; however, invasion of these cells was decreased by twofold when this operon was absent (Forman et al., 2008), implicating a ligand or enzymic function, although trimeric autotransporters are not known to have enzymic function (Leo et al., 2012).
Deletion of yadBC did not affect lethality of Y. pestis in pneumonic plague (Forman et al., 2008) and resulted in only mild attenuation in bubonic plague (Forman et al., 2008 and modified in 2013). However, yadB and yadC showed elevated expression in infected fleas (Vadyvaloo et al., 2010), raising the possibility that YadB and YadC function as transmission factors. In this study, we sought to identify phenotypes for yadBC, in fleas and in mouse models of bubonic and systemic plague. We report that growth temperature oppositely affects the previously documented ability of yadBC to promote entry into mammalian cells and a new phenotype that we describe herein. The data are consistent with a role of yadBC in skin early after transmission.
Methods
Bacterial strains and cultivation.
Bacterial strains used in this study are described in Table 1. Escherichia coli DH5α (Life Technologies) was used to maintain the pPCP2 : : Kan plasmid described previously (Forman et al., 2008) and was grown in Luria–Bertani broth or agar (Miller, 1972) at 37 °C.
Table 1. Strains and plasmids used in this study.
| Bacterial strain/plasmid | Key properties* | Source/reference |
| Strain | ||
| Y. pestis CO92 | ||
| CO92.S2 | yadBC+ pgm+ pCD2− (Lcr−) pFraΔcaf1 (F1−) pPCP2† (Pla+) | Forman et al. (2008) |
| CO92.S8 | ΔyadBC pgm+ pCD2− (Lcr−) pFra (F1+) pPCP2† (Pla+) | Forman et al. (2008) |
| CO92.S10 | ΔyadBC pgm+ pCD2− (Lcr−) pFra Δcaf1 (F1−) pPCP2† (Pla+) | Forman et al. (2008) |
| CO92.S15 | ΔyadBC/yadBC+ pgm+ pCD2− (Lcr−) pFra (F1+) pPCP2† (Pla+) | Forman et al. (2008) |
| CO92.S38 | ΔyadBC Δpgm (Pgm−) pCD2Ap (Lcr+) pFra (F1+) pPCP2 : : Kan (Pla+); spontaneous excisant of the pgm locus from strain CO92.S8 into which the Lcr plasmid from strain CO99-3015.S6 (Forman et al., 2008) was electroporated and the resident pPCP2 plasmid† was replaced by pPCP2 : : Kan by electroporation and homogenotization | This study |
| CO92.S39 | ΔyadBC Δpgm (Pgm−) pCD2Ap (Lcr+) pFra (F1+) pPCP2 : : Kan PlaS99A (Pla−); spontaneous excisant of the pgm locus from strain CO92.S8 into which the Lcr plasmid from strain CO99-3015.S6 (Forman et al., 2008) was electroporated and the resident pPCP2 plasmid† was replaced by pPCP2 : : Kan Pla S99A by electroporation and homogenotization | This study |
| CO92.S42 | ΔyadBC/yadBC+ Δpgm (Pgm−) pCD2Ap (Lcr+) pFra (F1+) pPCP2 : : Kan (Pla+); spontaneous excisant of the pgm locus from strain CO92.S15 into which the Lcr plasmid from strain CO99-3015.S6 (Forman et al., 2008) was electroporated and the resident pPCP2 plasmid† was replaced by pPCP2 : : Kan by electroporation and homogenotization | This study |
| CO92.S43 | ΔyadBC/yadBC+ Δpgm (Pgm−) pCD2Ap (Lcr+) pFra (F1+) pPCP2 : : Kan PlaS99A (Pla−); spontaneous excisant of the pgm locus from strain CO92.S8 into which the Lcr plasmid from strain CO99-3015.S6 (Forman et al., 2008) was electroporated and the resident pPCP2 plasmid† was replaced by pPCP2 : : Kan Pla S99A by electroporation and homogenotization | This study |
| E. coli | ||
| DH5α | F− Δ ϕ80d lacZΔM15 endA1 recA1 hsdR17(rm− mk+) supE44 thi-1 gyrA96 Δ (lacZYA-argF)U169; cloning host | Life Technologies |
| Plasmid | ||
| pPCP2 : : Kan† | 10.6 kb, Kmr; kan gene from pKD4 inserted into pPCP2 at bp 2135/2221 in the intergenic region downstream from the IS100 ATP-binding protein (YPPCP1.02); the construction deleted sequence between bp 2135 and 2221 | Forman et al. (2008) |
| pPlaS99A | pla with site mutation S99A in pSE380; Pla lacks proteolytic activity | Kukkonen et al. (2001) |
| pPCP2 : : Kan PlaS99A | Derivative of pPCP2 : : Kan† in which BamHI/HindIII fragment of pla from pPlaS99A was exchanged for the native BamHI/HindIII fragment of pla in pPCP2 : : Kan; Kmr | This study |
The native virulence plasmids of Y. pestis are the 9.6 kb pPCP (encoding the protease Pla), the 70.3 kb Lcr plasmid pCD (encoding the Ysc T3SS and Yops), and the 96.2 kb pFra (also called pMT; encoding the capsular fibril F1) (Perry & Fetherston, 1997). See Methods for additional information.
The native pPCP plasmid in Y. pestis CO92 is here designated pPCP2, reflecting the small differences between it and the pPCP plasmid in Y. pestis KIM, which is called pPCP1 (Hu et al., 1998; Parkhill et al., 2001).
Y. pestis has three native plasmids, the 96.2 kb pFra/pMT plasmid that encodes an abundant anti-phagocytic pilus Caf1 (also referred to as F1), the 70.3 kb ‘low-calcium response’ (Lcr) plasmid pCD that carries genes for the T3SS and transported effector proteins called Yops, and the 9.6 kb pPst/pPCP plasmid that encodes the surface protease Pla (Parkhill et al., 2001; Perry & Fetherston, 1997). All of these key genes are virulence properties and are maximally expressed at 37 °C. On the chromosome, there is an approximately 102 bp pgm locus that specifies the Ybt siderophore-based iron acquisition system that is needed for virulence of Y. pestis in peripheral tissues such as skin. Also present is part of the Hms exopolysaccharide-biosynthetic operon that functions at ambient temperature for biofilm formation and colonization of the flea vector. Strains that possess both a pgm locus and an Lcr plasmid are category A select agents; however, all strains used in this study were either Δpgm or Lcr− due to absence of the pCD plasmid. A strain lacking the pgm locus (Δpgm) is conditionally virulent: avirulent in peripheral tissues but essentially fully virulent from an intravenous route of infection. However, such strains grown on iron-replete medium can survive short periods in peripheral tissues, and that feature was exploited for short-term survival studies in skin.
Y. pestis strains were routinely grown to exponential phase at 28 or 37 °C in heart infusion broth (HIB; Difco Laboratories) supplemented with 2.5 mM CaCl2 and 0.2 % (w/v) xylose (sHIB) and on sHIB agar. For many experiments, Y. pestis was grown at 28 °C and shifted to 37 °C for 3 h for thermal induction of gene expression (denoted as 28 °C/37 °C3h). Where appropriate, carbenicillin (50 µg ml−1) or kanamycin (Km; 50 µg ml−1) were added to cultures for retention of plasmids. The presence of the pgm locus was confirmed by the formation of red colonies on Congo red agar (Surgalla & Beesley, 1969) at 28 °C. When the Lcr virulence plasmid pCD2Ap was introduced, strains were confirmed to show the Lcr-related absence of growth at 37 °C on HIB agar containing 0.2 % xylose, no added calcium, 0.2 M MgCl2 and 0.2 M sodium oxalate (MgOx plates) and calcium-dependent expression and secretion of Yops (Perry & Fetherston, 1997) in the defined medium TMH (Straley & Bowmer, 1986). Cell growth was monitored on a Spectronic Genesys 5 spectrophotometer at 620 nm.
Construction of Y. pestis strains.
The deletion of caf1 that encodes the subunit of the F1 fibril encompassed the entire caf1 coding sequence and 12 bp upstream. Because F1 is abundantly expressed at 37 °C and interferes with the interaction of the bacteria and mammalian cells (Du et al., 2002), Δcaf1 (F1−) strains were used for in vitro uptake studies with bacteria grown at 37 °C. F1+ strains were used for intradermal infection of mice.
Strains having derivatives of pPCP2 tagged with Kmr (pPCP2 : : Kan) or this plasmid expressing a proteolytically inactive Pla due to an amino acid substitution in Pla (S99A) were made by electroporation followed by selection for Kmr and homogenotization (Table 1). After growth at 28 °C for about 25 generations in sHIB+Km, the bacteria were spread on sHIB+Km agar, and colonies were analysed for their pPCP plasmid profile in agarose gels. The absence of pPCP2 from strains containing pPCP2 : : Kan plasmids was verified by PCR using primers for sequences flanking the Kan insertion in pPCP2: KD1-A, bp 2087-5′CAATCAGAACAGCAGGCCATGAACGACTGACAACATTACGAATATAAAATTGTGTAGGCTGGAGCTGCTTC3′; and KD2-B, bp 2266-5′CACTCTTCCGCTAATCCTGCATCATCGTGACATGTATCTGTACTCACATATGAATATCCTCCTTAGTTC3′. If the isolate had the npt (Kan) insertion in pPCP2, the band size was ~1000 bp; without the insertion, the band size was ~100 bp. Absence of Pla activity in the strain containing pPCP2 : : Kan PlaS99A was confirmed by the failure to lyse a fibrin film when tested alongside known Pla+ and Pla− strains. Fibrin films were prepared in Petri dishes as previously described by Beesley et al. (1967). For these tests, three colonies were suspended in 20 µl HIB, and 10 µl was spotted on a fibrin film. The plate was incubated overnight at 37 °C and observed for lysis. Pla protein expression from the pPCP2 derivatives was assayed in immunoblots prepared from whole bacterial cells grown at 28 °C. The blots were probed with rabbit anti-Pla antibody (kind gift of Jon D. Goguen, University of Massachusetts) and developed with alkaline phosphatase detection using nitro-blue tetrazolium and 5-bromo-4-chloro-3′-indolyphosphate (Sigma-Aldrich). The same amounts of Pla protein were expressed from pPCP2 : : Kan and pPCP2 : : Kan PlaS99A (data not shown).
The Δpgm Y. pestis strains CO92.S38 and CO92.S42 were obtained from the respective Pgm+ ancestors by spreading 500–1000 c.f.u. on several Congo red plates and screening for spontaneous excisants (white colonies) (Surgalla & Beesley, 1969). These were streak-purified and confirmed to be complete excisants for the pgm locus by PCR using Taq polymerase with the following primers: Common, bp 2135440-5′GCCAGACGGACCATCCAGTATATTGTAACGA3′; Reverse-1, bp 2137420-5′CCGCGCAGAGATTGTAATGAACCAGT3′; and Reverse-2. bp 2238231-5′CCCCCGCCAGATCCTTACCTTTAGATTGTA3′. Primers Common plus Reverse-1 give a 2009 bp product from genomic DNA that contains the pgm locus and no product from Δpgm DNA. Primers Common plus Reverse-2 give a 1961 bp product from genomic DNA that has undergone a complete deletion of the pgm locus and no product (102 791 bp: too large for Taq DNA polymerase) from DNA containing the pgm locus. Thermocycler conditions were as follows: one cycle for 5 min at 94 °C, 30 cycles for 40 min at 94 °C, 30 cycles for 40 min at 50 °C, 30 cycles for 45 min at 72 °C, one cycle for 5 min at 72 °C, then cooling to 4 °C. Known Pgm+ and Δpgm strains were analysed alongside the test strains as positive and negative controls. After confirmation that strains were truly Δpgm, the Lcr plasmid pCD2Ap was introduced by electroporation without losing exemption from select agent regulations.
Uptake by monocyte/macrophage-like cells.
J774A.1 mouse monocyte/macrophage-like cells (ATCC) were grown to ~95 % confluency in six-well culture dishes. Thirty minutes prior to infection, the wells were washed twice with serum-free medium (RPMI1640; Life Technologies or Sigma-Aldrich) and incubated in serum-free medium until the infection. Overnight cultures of Y. pestis grown at 28 or 37 °C were diluted into serum-free culture media, and 1 ml aliquots were added to each of three wells for a nominal m.o.i. of 10. Actual m.o.i. values were determined by plating 2–40 c.f.u. After 5 min of centrifugation at 500 g to facilitate a synchronous contact, the cells were incubated in a CO2 incubator for 15 min or 1 h at 37 °C for adherence or invasion assays, respectively. To measure gentamicin-protected c.f.u. (intracellular bacteria), gentamicin (30 µg ml−1) was added to the wells and the plate was incubated for an additional 60 min. The cells were then washed twice with PBS, subjected to water lysis and plated. Results are shown as the percentage of the output c.f.u. relative to the input c.f.u. from triplicate wells for each experiment.
Infection of fleas.
Equal numbers of male and female Oriental rat fleas (Xenopsylla cheopsis) were infected with Y. pestis CO92.S15 (Pgm+ Lcr− reconstituted yadBC+) or CO92.S8 (Pgm+ Lcr− ΔyadBC) in fresh heparinized mouse blood as described previously (Hinnebusch et al., 1996). The bacteria had been incubated overnight in brain HIB (Difco) at 37 °C without aeration, suspended in PBS, enumerated by direct counting in a Petroff-Hausser chamber and added to give 5×108 c.f.u. (ml blood)–1. The infected fleas were held at 21 °C and 75 % relative humidity, fed twice weekly on non-infected mice and observed for blockage as described in detail previously (Hinnebusch et al., 1996). Bacterial suspensions were obtained from about 30 fleas per strain after 1 h and 28 days and plated for c.f.u. counts on Yersinia selective agar (Difco) as described previously (Hinnebusch et al., 2002).
Infection of mice: virulence tests.
All experiments with mice were reviewed and approved by the University of Kentucky Institutional Animal Care and Use Committee. Mice used were 6–8-week-old female C57BL/6N.HSD mice (Harlan Sprague–Dawley). CO92.S38 and reconstituted ΔyadBC/yadBC+ Y. pestis CO92.S42 strains were compared for their virulence in a mouse model of systemic plague under Animal Biosafety Level 2 conditions. The yersiniae were grown as noted at 37, 28 or 28 °C/37 °C3h. Groups of four mice were anaesthetized with isoflurane using a rodent anaesthesia machine and were infected intravenously in the retro-orbital plexus with bacteria suspended in 100 µl of non-pyrogenic sterile 0.9 % NaCl solution delivered from a tuberculin syringe fitted with a half-inch 27-gauge needle. To test the lethality of the infection, the infection dose was 1300 (≥10-fold above the LD50 of the yadBC+ strain) and the mice were then observed twice daily for signs of disease for up to 14 days and were humanely killed when morbibund. To assess effects of yadBC on early colonization of liver and spleen in systemic plague, the infection dose was 2×104, and c.f.u. were determined in liver and spleen at 3 h post-infection. A dose of approximately 400 was used for infections where c.f.u. were determined daily for the first 3 days post-infection. Actual doses given to mice were determined by plating for c.f.u. as previously described (e.g. Ye et al., 2011).
Polymorphonuclear leukocyte (PMN) ablation.
PMNs were depleted by two intraperitoneal injections of 200 µg anti-Ly6G antibody (clone 1A8; Bio X Cell) in 0.1 ml PBS, 24 h before infection and just prior to infection. Mock-treated mice similarly received rat IgG (Sigma-Aldrich). To assess the amount of depletion, spleens of mice infected intradermally for 3 h were removed, splenocytes were recovered, and PMNs were distinguished and quantified as described previously (Ye et al., 2011). FcBlock (rat anti-mouse CD16/CD32) and the following fluorochrome-coupled antibodies obtained from BD Pharmingen were used to distinguish PMNs in the splenic leukocyte population: fluorescein isothiocyanate-conjugated anti-CD11b and phycoerythrin-conjugated anti-Ly6G. Splenocytes were treated with FcBlock on ice for 15 min and were then incubated with a mixture of the fluorochrome-coupled antibodies for 30 min on ice. The cells were washed with ice-cold PBS and suspended in ice-cold PBS containing freshly prepared 4 % (w/v) paraformaldehyde (pH 7.4) for at least 30 min. Flow cytometric analysis was carried out using a BD Biosciences LSRII flow cytometer. Dead cells and debris were gated using scatter at 355 nm (UV). The data were analysed by FlowJo software (Version 7.6.1; Tree Star). PMNs comprised the Ly6G+ CD11b+ UV− cells and were found to be depleted by 94–97 % by the anti-Ly6G treatment.
Infection of mice: recovery of bacteria or RNA from intradermally infected skin.
Groups of mice were shaved on the back around the base of the tail and were lightly anaesthetized with isoflurane to effect. One hundred microlitres of the bacterial suspension was injected intradermally at the base of the tail from a tuberculin syringe fitted with a half-inch 27-gauge needle. Three hours later a patch of skin approximately 1.5 cm in diameter surrounding the injection site was removed from each mouse and placed in 2 ml cold PBS in a Miltenyi Biotech dissociator M tube. Each skin piece was then dissociated for 90 s using the Miltenyi GentleMACS dissociator set on the strongest program (RNA.02). Collagenase stock (20 µl in PBS at 40 000 U ml−1) and 11 µl of dispase stock (in PBS at 224 U ml−1) were added, and the tubes were incubated for 30 min at 37 °C. The samples were then given a second dissociation treatment. The resulting suspensions were diluted in freshly made pH 11.0 water, and samples of three or four dilutions were spread on duplicate sHIB plates for c.f.u. determination.
To obtain RNA from infected skin, patches of skin recovered as described above were placed in weighed 15 ml Miltenyi M dissociator tubes containing 4 ml cold buffer [1 vol. RLT buffer (Qiagen) plus 0.01 vol. 100 % β-mercaptoethanol]. The tubes were then reweighed and the skin dissociated using the GentleMACS dissociator program RNA.01. RNase free water (8 ml) was added per tube, then proteinase K was added to give 0.02 mg ml−1, and the tubes were incubated at 55 °C for 20 min. They were centrifuged for 5 min at 3000 g, and the supernatant was passed through a 40 µm mesh nylon filter into a 50 ml polypropylene conical tube. Then, 6 ml of 100 % ethanol was mixed in, and the sample was immediately processed to obtain RNA by using a Qiagen Midi-kit according the manufacturer’s instructions. The sample weights were used to normalize RNA amounts on a tissue weight basis.
Relative quantification of mRNA.
Net expression of genes for chemokines and the PMN surface marker Ly6G in infected mouse skin was measured relative to UbC (encoding ubiquitin C) by two-step quantitative real-time polymerase chain reaction (RT-PCR) using SYBR Green detection. Primer sequences were obtained from the RTPrimer Database (www.rtprimerdb.org).
Statistics.
Except where noted, experiments were performed two or more times. Student’s unpaired two-tailed t-test was used to determine the significance of differences.
Results
yadBC-associated enhanced uptake of Y. pestis by J774A.1 cells is temperature-dependent
The presence of the yadBC operon has been shown to promote invasion of epithelioid cells (Forman et al., 2008). The invasion phenotype of yadBC might provide a protected niche and contribute to the early survival advantage of 37 °C-grown yadBC+ Y. pestis in liver and spleen during systemic plague or 28 °C-grown Y. pestis in skin. Accordingly, we tested whether yadBC would promote uptake by monocyte/macrophage-like cells. YadB and YadC were expressed from the native chromosomal operon or were absent due to deletion of the operon. The yersiniae were grown at 37 °C as in previous studies (Forman et al., 2008) or grown at 28 °C. Table 2 shows that when the bacteria had been grown at 37 °C, the presence of yadBC correlated with a 60 % increase in uptake of the bacteria. However, no significant difference was seen in uptake of yadBC+ and ΔyadBC Y. pestis when the bacteria had been grown at 28 °C. This revealed a temperature effect in the phenotype of the yadBC operon, such that the enhanced bacterial uptake would not be operating immediately after delivery of the bacteria by flea-bite.
Table 2. yadBC-dependent modulation of bacterial uptake by J774A.1 cells.
| Test and bacterial growth temperature (no. of experiments) | Y. pestis CO92 strain; key properties | Percentage invasion (mean±sd) |
| Adherence to J774A.1 | ||
| 1 37 °C (2) | CO92.S10; Pla+F1− ΔyadBC | 94±14 |
| CO92.S2; Pla+F1− native yadBC+ | 74±22 (P = 0.0891) | |
| Intracellular, J774A.1 | ||
| 2 37 °C (2) | CO92.S10; Pla+F1− ΔyadBC | 54±21 |
| CO92.S2; Pla+ F1− native yadBC+ | 87±13 (P = 0.0190) | |
| 3 28 °C (3) | CO92.S10 Pla+F1− ΔyadBC | 28±21 |
| CO92.S2 Pla+F1− native yadBC+ | 31±16 (P = 0.748) | |
YadB and YadC are not required for lethality of systemic plague or early bacterial growth and survival in liver and spleen
Previous studies have shown that yadBC is not required for lethality of pneumonic plague (due to intranasal infection) (Forman et al., 2008). However, we had not tested for a potential role of yadBC in systemic plague, where the bacteria colonize liver and spleen following either lung or skin infection. In this situation, the bacteria would be pre-adapted to mammalian body temperature, and the yadBC-promoted uptake property at 37 °C might be relevant. For these in vivo tests the Y. pestis strains needed to have the Lcr virulence plasmid (see Methods); accordingly, we used the Lcr+ ΔyadBC Y. pestis CO92.S38 and the yadBC-reconstituted Lcr+ strain CO92.S42. The yersiniae were grown at 28 °C and shifted to 37 °C for 3 h prior to intravenous infection of mice. In one test of lethality in which the mice were given 1300–1400 bacteria, all of the mice died and the presence of yadBC did not have a significant effect on how long they survived (data not shown). Likewise, when viable bacterial numbers in organs were measured following infection of mice with 400 organisms, there was no effect of the presence of yadBC on growth in liver and spleen as early as 1 day post-infection (Fig. 1). We further tested whether yadBC promotes very early colonization by measuring recovery of viable bacteria from liver and spleen 3 h after intravenous infection. Fig. 2 shows that similar recoveries were obtained whether the infecting strain was yadBC+ or ΔyadBC. These data showed that YadB and YadC do not confer a unique survival advantage in the systemic plague model for Y. pestis.
Fig. 1.
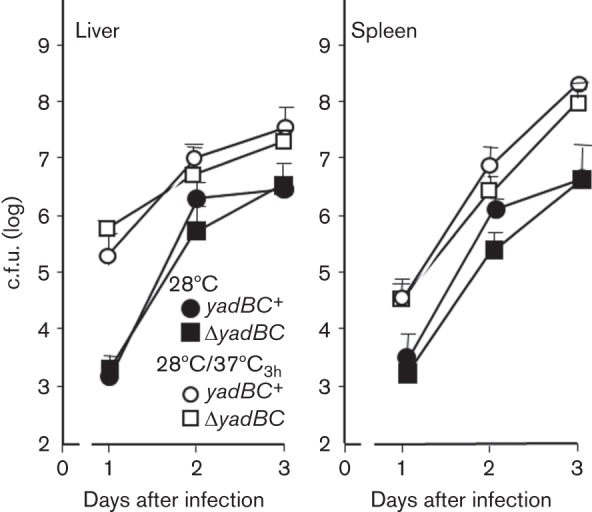
yadBC does not affect infection dynamics in systemic plague. Y. pestis strains CO92.S38 (ΔyadBC; otherwise conditionally virulent; squares) and CO92.S42 (yadBC+ by restoration of the native yadBC operon; otherwise conditionally virulent; circles) were grown either at 28 °C (solid symbols) or at 28 °C and shifted to 37 °C for 3 h (open symbols). Groups of mice were infected intravenously in the retro-orbital plexus with 400 c.f.u. and were analysed for bacterial burdens in liver and spleen on the indicated days post-infection. The data were pooled and averaged from two experiments and represent between six and eight mice per datum point.
Fig. 2.
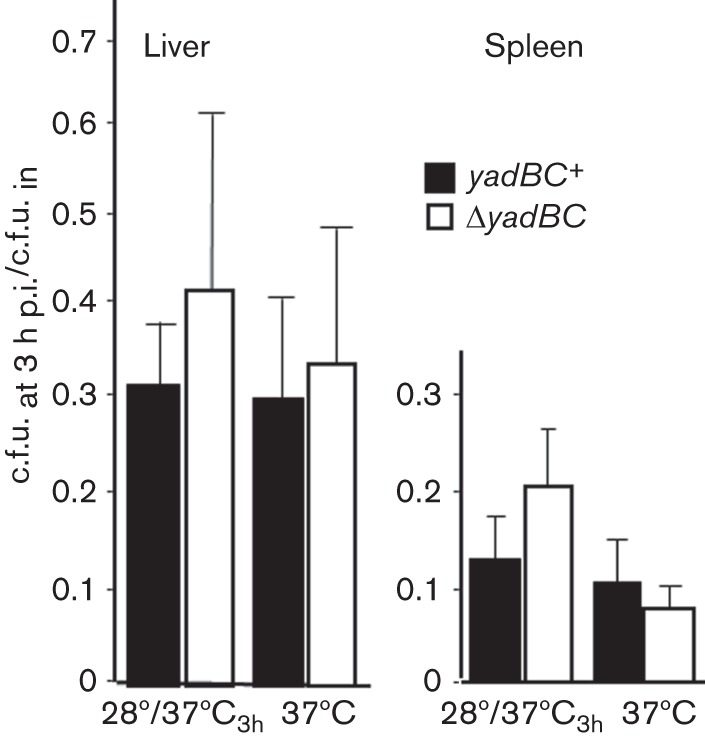
YadB and YadC do not affect early survival of Y. pestis in systemic plague. Pgm− Lcr+ Y. pestis strains CO92.S38 (ΔyadBC; open bars) and CO92.S42 (yadBC+ by restoration of the native yadBC operon; solid bars) were grown as indicated to induce expression of thermally regulated proteins 3 h prior to infecting mice by the intravenous route. Groups of mice were infected intravenously with doses ranging from 5×103 to 104 bacteria. After 3 h, the recovery of viable bacteria was determined in livers and spleens and is presented as the fraction of input c.f.u. that was recovered. The data for the 28 °C/37 °C3h growth protocol are pooled from four replicate experiments, and each datum point represents the mean±sd for 14 mice. Two experiments were done for yersiniae grown at 37 °C, and each datum point represents the mean±sd of pooled data from seven mice.
yadBC does not promote colonization of fleas but does confer an early phenotype in skin
A microarray study of Y. pestis gene expression in fleas compared with in vitro at 21 °C found that both yadB and yadC were more highly expressed in the flea than in vitro (Vadyvaloo et al., 2010). Accordingly, YadB and YadC might have roles in colonization of the flea vector at ambient temperature. This was tested by comparing the ability of Pgm+ Lcr− ΔyadBC Y. pestis CO92.S8 and the yadBC-reconstituted Y. pestis CO92.S15 to colonize fleas. The two strains blocked fleas equivalently and exhibited similar infection rates and bacterial loads (Table 3). Accordingly, YadB and YadC do not play a significant role in colonization of the flea vector.
Table 3. Colonization of fleas (Xenopsylla cheopsis) by Pgm+* Lcr− Y. pestis differing in presence of yadBC.
| Y. pestis strain used for infecting fleas | c.f.u. ml−1 in blood meal | Percentage fleas blocked† | Percentage fleas infected after: | Average no. of Y. pestis c.f.u. per flea after: | ||
| 1 h | 28 days | 1 h | 28 days | |||
| CO92.S8 (Lcr−) ΔyadBC | 5.7×108 | 40 | 100 % | 83 % | 2.5×104 | 6.1×105 |
| CO92.S15 (Lcr−) ΔyadBC/yadBC+ | 5.1×108 | 38 | 100 % | 70 % | 5.8×104 | 8.5×105 |
The pgm locus is required for efficient colonization of the flea; the virulence genes on the Lcr plasmid are not (see Methods).
In total, n = 105 fleas infected with mutant and 110 fleas infected with complemented mutant, with roughly equal numbers of males and females (the percentage blocked represents the cumulative number that became blocked during the 4-week observation period).
However, they might serve as a transmission factor: the higher yadBC expression in fleas might provide a protective effect during the first hours after transmission by flea-bite. We were unable to do actual flea transmission studies; accordingly, this idea was tested by measuring recovery of ΔyadBC and yadBC-reconstituted (ΔyadBC/yadBC+) Y. pestis strains 3 h after intradermal infection of mice. Fig. 3 shows that all yadBC+ strains were recovered significantly better than the ΔyadBC mutants from skin when the yersiniae had been grown at 28 °C. As anticipated for bacteria pre-grown at 28 °C, the absence of Pla’s proteolytic activity did not have a significant effect on the ability of yadBC to support early survival in skin. The data also suggest that the Lcr plasmid (as expected) and pgm loci are not involved for bacteria grown at 28 °C, although these two properties were not tested singly. However, when the bacteria had been given a 3 h incubation at 37 °C, both ΔyadBC and yadBC+ strains were recovered with high efficiency (not significantly different from that for the yadBC+ strain grown at 28 °C). These findings showed that a survival advantage is conferred by yadBC in skin early after infection by Y. pestis grown at ambient temperature. After incubation for 3 h at 37 °C, evidently a thermally induced property compensates for the absence of yadBC, and yadBC is not needed.
Fig. 3.
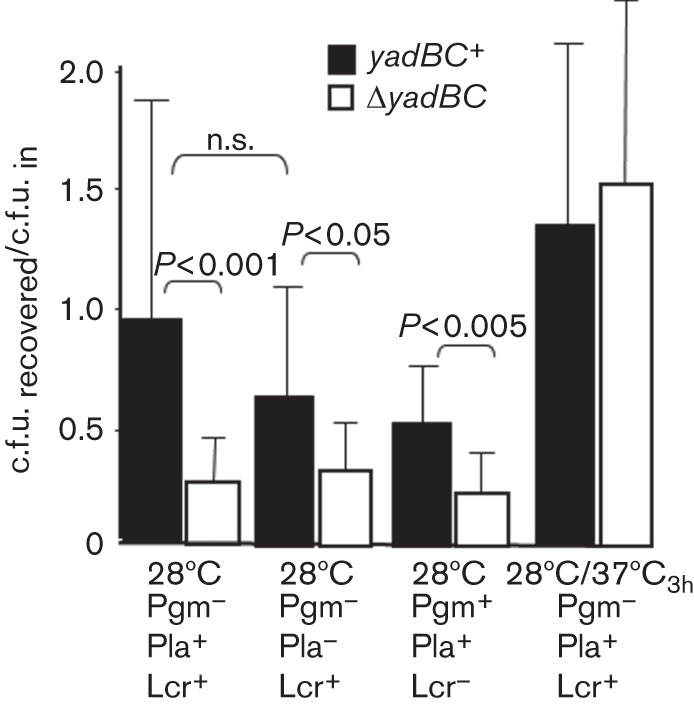
yadBC confers a phenotype in skin. Pgm− Lcr+ Y. pestis strains differing in the presence of the yadBC operon and activity of the Pla protease (CO92.S38 and CO92.S42; CO92.S39 and CO92.S43) were grown at 28 °C, and doses of approximately 5×103 bacteria were injected intradermally into C57BL/6 mice. For comparison, the Pla+ pair of strains also was given 3 h at 37 °C prior to infection (28 °C/37 °C3h). After 3 h, the infected skin was removed, homogenized and plated for viable bacterial numbers. Two additional ΔyadBC/yadBC+ pairs were grown at 28 °C and tested for recovery to assess the importance of the Lcr plasmid and pgm locus on the yadBC phenotype. These were the Pgm+ Lcr− Pla+ strains CO92.S15 and CO92.S8 and the Pgm− Lcr− Pla− strains CO92.S39 and CO92.S43. The recovery of viable numbers was normalized to the input dose for each strain and is presented as mean±sd. Open bars, ΔyadBC Y. pestis; closed bars, reconstituted strain (ΔyadBC/yadBC+). The data were pooled from multiple experiments: 4–6 experiments (14–23 mice per datum point) for the Pgm− strains grown at 28 °C, two experiments (eight mice per datum point) for the Pgm+ strains, and four experiments (14–16 mice per datum point) for the Pgm− strains grown at 28 °C/37 °C3h. Statistically significant differences are indicated; n.s., not statistically significantly different.
The pro-survival effect of yadBC correlates with low expression of the chemokine CXCL1
We wondered whether the early survival advantage conferred by yadBC in skin might be related to protection of the bacteria against PMNs, which are known to be able to kill Y. pestis. Accordingly, infection experiments like those of Fig. 3 were conducted with mice ablated of PMNs by treatment with an antibody against the PMN-specific surface marker Ly6G. Fig. 4(a) shows that ablation of PMNs appeared selectively to enhance recovery of ΔyadBC Y. pestis; however, owing to the large scatter in the data for these experiments, the difference from data for mock-treated mice was not statistically significant. Because the hypothesis had not been ruled out conclusively, we modified the assay and tested whether recruitment of PMNs was affected by the presence of yadBC. To measure relative numbers of PMNs, we used RT-PCR to determine net message abundance for the Ly6G surface marker specific to PMNs (normalized to expression of the ubiquitin C gene, UbC) in infected skin. We also quantified the relative abundances of messages for key chemokines involved in recruitment of PMNs, namely CXCL1 (KC) and CXCL2 (MIP-2). Fig. 4(b) shows that the differences found for Ly6G message were not statistically significant; however, if yadBC was missing there was a greater abundance of mRNA for the potent chemokine CXCL1. Expression of net message for CXCL2 was not affected by the presence of yadBC in the infecting strain. (Cxcl2 message was not detected in skin from uninfected mice.) These data point to an effect of YadB and/or YadC on resident cells in skin that express Cxcl1 when stimulated. The findings are consistent with an early role of yadBC in creating a safe niche by delaying inflammation while the bacteria adapt to the mammalian environment.
Fig. 4.
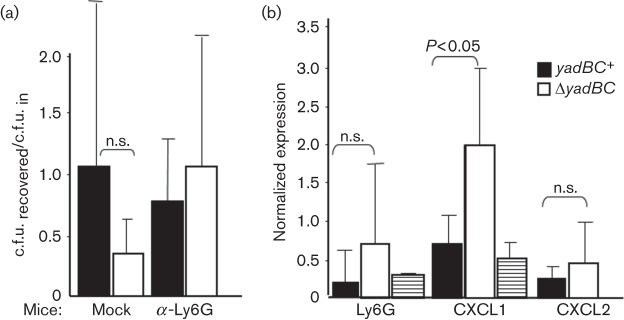
yadBC may dampen recruitment of PMNs. (a) Groups of mice were infected intradermally for 3 h with approximately 2×103 Pgm− Lcr+ ΔyadBC Y. pestis CO92.S38 and yadBC-reconstituted CO92.S42 strain grown at 28 °C (open and closed bars, respectively). After 3 h, the infected skin was removed, dissociated and plated for c.f.u. The recovery of viable numbers was normalized to the input dose for each strain and is presented as the mean±sd. The mock and α-Ly6G mice were given, respectively, 200 µg of rat IgG (mock ablation) or anti-Ly6G (to ablate PMNs) intraperitoneally 24 h prior to infection and immediately prior to infection. The bars represent 16 anti-Ly6G-treated and nine mock-treated mice infected with ΔyadBC Y. pestis CO92.S38 and 14 infected anti-Ly6G-treated and 10 mock-treated mice infected with Y. pestis CO92.S42. (b) Groups of three mice were infected intradermally for 3 h with approximately 2×103 Pgm− Lcr+ ΔyadBC Y. pestis CO92.S38 or yadBC+-reconstituted CO92.S42 bacteria grown at 28 °C (open and closed bars, respectively), and message abundance for the PMN marker Ly6G and the chemokines CXCL1 and CXCL2 were determined by RT-PCR in the dissociated skin and normalized to the message level of UbC (ubiquitin C). Skin from two uninfected mice (striped bars) was similarly analysed. The data were pooled from two experiments (six mice per datum point, except for uninfected mice, for which data from one mouse from each of the two experiments were pooled). Data are given as mean±sd. Statistically significant differences are indicated; n.s., not statistically significantly different.
Discussion
yadBC was hypothesized to represent a pathoadaptive acquisition for vector-borne transmission of plague (Forman et al., 2008), but it has not been found to have a major effect on lethality of plague (Forman et al., 2008 and modified in 2013). It is well known that virulence proteins may not be essential for lethality but still play important roles in the infection or disease processes; and YadB and YadC are located at the bacterial surface where many host–pathogen interactions take place. Therefore, we believed that further investigation of yadBC function was needed. We found that the roughly twofold enhancement of invasion previously found for epithelioid cells also applied to uptake of the bacteria by macrophage-like cells. These cells are believed to be a crucial protective intracellular niche for Y. pestis and are a major cell type that interacts with the bacteria in systemic plague (Lukaszewski et al., 2005; Marketon et al. 2005; Pujol & Bliska 2005; Ye et al., 2009). However, we found no evidence of a critical role for yadBC in promoting early colonization or growth in organs in systemic plague. A further consideration is that the major invasin so far found for Y. pestis is the surface protease Pla. The ability of Pla to promote invasion of Y. pestis is strongly temperature-regulated, being about 17-fold greater at 37 °C than at 26 °C (Cowan et al., 2000). This is probably due to both greatly increased amounts of Pla protein and higher Pla-specific activity at 37 °C (Chromy et al., 2005; McDonough & Falkow, 1989; Motin et al., 2004; Suomalainen et al., 2010). The effect of YadB and YadC on invasion is small compared with that of Pla, perhaps explaining why loss of yadBC did not noticeably impact colonization and growth in the systemic plague model where Pla is strongly expressed. Given that yadBC is only weakly expressed and confers no adhesin activity, YadB and YadC themselves are unlikely candidates for invasins. We speculate that the effects of yadBC on bacterial uptake arise from small effects that YadB and YadC have on Pla, so that when Pla is essentially absent at 28 °C this effect of yadBC disappears.
The fact that yadBC transcripts are elevated in the flea (Vadyvaloo et al., 2010) prompted us to investigate whether yadBC serves a role in the flea or early after transmission by flea-bite. We found no effect of the absence of yadBC on flea colonization but did find that yadBC+ Y. pestis could be recovered from infected skin in numbers approaching the input doses, in contrast to ΔyadBC bacteria, which were recovered in two- to fourfold lower amounts. This implies that yadBC was expressed in the input bacteria grown at 28 °C in vitro or in skin immediately after infection. Current evidence indicates that the operon is at least weakly expressed under these conditions. Previously, the yadBC promoter in Y. pestis grown at 26 °C was found to be active but two- to threefold lower than at 37 °C (measured from a promoter–lacZ fusion in Forman et al., 2008). Our measurements of yadC net message abundance by quantitative RT-PCR on the native operon for Y. pestis in exponential or stationary phase at 28 and 37 °C showed that yadC is weakly expressed under all of these conditions (Table S1 available in Microbiology Online). Furthermore, YadC immunization from a live Salmonella vaccine elicited partial protection against 28 °C-grown fully virulent Y. pestis CO92 from the subcutaneous route of infection, implying that YadC is present on the surface of Y. pestis after growth at 28 °C and injection into skin (Sun et al., 2013). We do not yet know the basis of the effect of YadB and YadC on recoverability from skin. Pla and the Lcr plasmid did not appear to be involved, not surprisingly as this effect of YadB and YadC was seen only for bacteria grown at 28 °C. The pgm locus also did not appear to be involved (Fig. 3). Lower recovery in the absence of yadBC could be due to increased recruitment of phagocytes, increased killing by phagocytes, spread of ΔyadBC bacteria beyond the sampled area of skin, bacterial aggregation or tight adherence to matrix structures lost during preparation. We believe that increased killing by phagocytes is not involved, because comparisons of survival of yadBC+ and ΔyadBC Y. pestis in casein-elicited mouse peritoneal cells (predominantly PMNs) found no significant differences (our unpublished data). Tests for the involvement of PMNs by ablating them prior to infection of skin or measuring levels of mRNA for a PMN marker were suggestive but equivocal, whereas assays for mRNA for the PMN chemoattractant CXCL1/KC revealed about fourfold higher levels when the infecting bacteria lacked yadBC. In contrast, no difference was seen for mRNA for another major PMN chemoattractant, CXCL2. Both of these chemokines bind to the same receptor (CXCR2) but can be produced at different times during the inflammatory response and by different cell types. In injured mouse skin, CXCL1 was produced by non-myeloid cells (endothelial cells and fibroblasts) within a few hours of injury whereas CXCL2 was made by infiltrating myeloid cells (monocytes and PMNs) predominantly later (Armstrong et al., 2004). This temporal pattern was also found in mouse lungs infected with Legionella (Tateda et al., 2001). The PMNs that are attracted to the focus of infection might act in a predominantly regulatory manner by modulating the cytokine milieu as in the intracellular pathogen Legionella (Tateda et al., 2001) or by killing the bacteria as in an extracellular pathogen, Pseudomonas (Tsai et al., 2000). The gene for CXCL1 is predominantly regulated at the level of transcription (Armstrong et al., 2004); accordingly, our finding of a yadBC-associated difference in Cxcl1 mRNA supports the hypothesis that YadB and/or YadC in some way influence production of this chemoattractant by resident non-myeloid cells early after infection of skin by Y. pestis. Given that YadB and YadC are predicted to be in the outer membrane, it is possible that they act indirectly by influencing availability or reactivity of another bacterial surface component such as the lipooligosaccharide.
When the yersiniae were pre-incubated for 3 h at 37 °C, both yadBC+ and ΔyadBC strains were recovered with high efficiency, indicating that a thermally induced property such as the T3SS and its secreted effector proteins called Yops or the capsular fibril F1, to name just a few, had replaced or removed any need for YadB and YadC. Accordingly, we hypothesize that YadB and/or YadC are transmission factors that are expressed during growth in the flea vector and promote early survival in skin.
Acknowledgements
This study was supported by PHS (NIAID) ARRA grant R21 AI083861, PHS (NIAID) grant R01 AI48491, and by a University of Kentucky Faculty Research Support grant, all to S. C. S. The authors acknowledge Christopher Jester (Department of Biology, University of Kentucky) for measuring survival of Y. pestis in PMNs. None of the authors has any conflict of interest that would affect this work.
Abbreviations:
- Km
kanamycin
- Lcr
‘low-calcium response’
- PMN
polymorphonuclear leukocyte
- RT-PCR
real-time polymerase chain reaction
- T3SS
type III secretion system
Footnotes
One supplementary table is available with the online version of this paper.
References
- Armstrong D. A., Major J. A., Chudyk A., Hamilton T. A. (2004). Neutrophil chemoattractant genes KC and MIP-2 are expressed in different cell populations at sites of surgical injury. J Leukoc Biol 75, 641–648. 10.1189/jlb.0803370 [DOI] [PubMed] [Google Scholar]
- Beesley E. D., Brubaker R. R., Janssen W. A., Surgalla M. J. (1967). Pesticins. 3. Expression of coagulase and mechanism of fibrinolysis. J Bacteriol 94, 19–26. 10.1189/jlb.0803370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouikha I., Hinnebusch B. J. (2012). Yersinia–flea interactions and the evolution of the arthropod-borne transmission route of plague. Curr Opin Microbiol 15, 239–246. 10.1016/j.mib.2012.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chromy B. A., Choi M. W., Murphy G. A., Gonzales A. D., Corzett C. H., Chang B. C., Fitch J. P., McCutchen-Maloney S. L. (2005). Proteomic characterization of Yersinia pestis virulence. J Bacteriol 187, 8172–8180. 10.1128/JB.187.23.8172-8180.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan C., Jones H. A., Kaya Y. H., Perry R. D., Straley S. C. (2000). Invasion of epithelial cells by Yersinia pestis: evidence for a Y. pestis-specific invasin. Infect Immun 68, 4523–4530. 10.1128/IAI.68.8.4523-4530.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Rosqvist R., Forsberg A. (2002). Role of fraction 1 antigen of Yersinia pestis in inhibition of phagocytosis. Infect Immun 70, 1453–1460. 10.1128/IAI.70.3.1453-1460.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felek S., Tsang T. M., Krukonis E. S. (2010). Three Yersinia pestis adhesins facilitate Yop delivery to eukaryotic cells and contribute to plague virulence. Infect Immun 78, 4134–4150. 10.1128/IAI.00167-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman S. F., Wulff C. R., Myers-Morales T., Cowan C., Perry R. D., Straley S. C. (2008). yadBC of Yersinia pestis, a new virulence determinant for bubonic plague. Infect Immun 76, 578–587. 10.1128/IAI.00219-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Zhou D., Pang X., Song Y., Zhang L., Bao J., Tong Z., Wang J., Guo Z. & other authors (2004). Microarray analysis of temperature-induced transcriptome of Yersinia pestis. Microbiol Immunol 48, 791–805. 10.1111/j.1348-0421.2004.tb03605.x [DOI] [PubMed] [Google Scholar]
- Hinnebusch B. J. (2005). The evolution of flea-borne transmission in Yersinia pestis. Curr Issues Mol Biol 7, 197–212. [PubMed] [Google Scholar]
- Hinnebusch B. J., Perry R. D., Schwan T. G. (1996). Role of the Yersinia pestis hemin storage (hms) locus in the transmission of plague by fleas. Science 273, 367–370. 10.1126/science.273.5273.367 [DOI] [PubMed] [Google Scholar]
- Hinnebusch B. J., Rosso M.-L., Schwan T. G., Carniel E. (2002). High-frequency conjugative transfer of antibiotic resistance genes to Yersinia pestis in the flea midgut. Mol Microbiol 46, 349–354. 10.1046/j.1365-2958.2002.03159.x [DOI] [PubMed] [Google Scholar]
- Hu P., Elliott J., McCready P., Skowronski E., Garnes J., Kobayashi A., Brubaker R. R., Garcia E. (1998). Structural organization of virulence-associated plasmids of Yersinia pestis. J Bacteriol 180, 5192–5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukkonen M., Lähteenmäki K., Suomalainen M., Kalkkinen N., Emödy L., Lång H., Korhonen T. K. (2001). Protein regions important for plasminogen activation and inactivation of α2-antiplasmin in the surface protease Pla of Yersinia pestis. Mol Microbiol 40, 1097–1111. 10.1046/j.1365-2958.2001.02451.x [DOI] [PubMed] [Google Scholar]
- Leo J. C., Grin I., Linke D. (2012). Type V secretion: mechanism(s) of autotransport through the bacterial outer membrane. Philos Trans R Soc Lond B Biol Sci 367, 1088–1101. 10.1098/rstb.2011.0208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaszewski R. A., Kenny D. J., Taylor R., Rees D. G., Hartley M. G., Oyston P. C. (2005). Pathogenesis of Yersinia pestis infection in BALB/c mice: effects on host macrophages and neutrophils. Infect Immun 73, 7142–7150. 10.1128/IAI.73.11.7142-7150.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marketon M. M., DePaolo R. W., DeBord K. L., Jabri B., Schneewind O. (2005). Plague bacteria target immune cells during infection. Science 309, 1739–1741. 10.1126/science.1114580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough K. A., Falkow S. (1989). A Yersinia pestis-specific DNA fragment encodes temperature-dependent coagulase and fibrinolysin-associated phenotypes. Mol Microbiol 3, 767–775. 10.1111/j.1365-2958.1989.tb00225.x [DOI] [PubMed] [Google Scholar]
- Miller J. H. (1972). Appendix I. Formulas and recipes. In Experiments in Molecular Genetics. Plainview, NY: Cold Spring Harbor Laboratory Press; p. 433. [Google Scholar]
- Montminy S. W., Khan N., McGrath S., Walkowicz M. J., Sharp F., Conlon J. E., Fukase K., Kusumoto S., Sweet C. & other authors (2006). Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat Immunol 7, 1066–1073. 10.1038/ni1386 [DOI] [PubMed] [Google Scholar]
- Motin V. L., Georgescu A. M., Fitch J. P., Gu P. P., Nelson D. O., Mabery S. L., Garnham J. B., Sokhansanj B. A., Ott L. L. & other authors (2004). Temporal global changes in gene expression during temperature transition in Yersinia pestis. J Bacteriol 186, 6298–6305. 10.1128/JB.186.18.6298-6305.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhill J. M., Wren B. W., Thomson N. R., Titball R. W., Holden M. T., Prentice M. B., Sebaihia M., James K. D., Churcher C. & other authors (2001). Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413, 523–527. 10.1038/35097083 [DOI] [PubMed] [Google Scholar]
- Perry R. D., Fetherston J. D. (1997). Yersinia pestis–etiologic agent of plague. Clin Microbiol Rev 10, 35–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol C., Bliska J. B. (2005). Turning Yersinia pathogenesis outside in: subversion of macrophage function by intracellular yersiniae. Clin Immunol 114, 216–226. 10.1016/j.clim.2004.07.013 [DOI] [PubMed] [Google Scholar]
- Sebbane F., Lemaître N., Sturdevant D. E., Rebeil R., Virtaneva K., Porcella S. F., Hinnebusch B. J. (2006). Adaptive response of Yersinia pestis to extracellular effectors of innate immunity during bubonic plague. Proc Natl Acad Sci U S A 103, 11766–11771. 10.1073/pnas.0601182103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodeinde O. A., Subrahmanyam Y. V., Stark K., Quan T., Bao Y., Goguen J. D. (1992). A surface protease and the invasive character of plague. Science 258, 1004–1007. 10.1126/science.1439793 [DOI] [PubMed] [Google Scholar]
- Straley S. C., Bowmer W. S. (1986). Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect Immun 51, 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W., Olinzock J., Wang S., Sanapala S., Curtiss R., III (2013). Evaluation of YadC protein delivered by live attenuated Salmonella as a vaccine against plague. Pathog Dis 69, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen M., Lobo L. A., Brandenburg K., Lindner B., Virkola R., Knirel Y. A., Anisimov A. P., Holst O., Korhonen T. K. (2010). Temperature-induced changes in the lipopolysaccharide of Yersinia pestis affect plasminogen activation by the pla surface protease. Infect Immun 78, 2644–2652. 10.1128/IAI.01329-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surgalla M. J., Beesley E. D. (1969). Congo red-agar plating medium for detecting pigmentation in Pasteurella pestis. Appl Microbiol 18, 834–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateda K., Moore T. A., Newstead M. W., Tsai W. C., Zeng X., Deng J. C., Chen G., Reddy R., Yamaguchi K., Standiford T. J. (2001). Chemokine-dependent neutrophil recruitment in a murine model of Legionella pneumonia: potential role of neutrophils as immunoregulatory cells. Infect Immun 69, 2017–2024. 10.1128/IAI.69.4.2017-2024.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai W. C., Strieter R. M., Mehrad B., Newstead M. W., Zeng X., Standiford T. J. (2000). CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect Immun 68, 4289–4296. 10.1128/IAI.68.7.4289-4296.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadyvaloo V., Jarrett C., Sturdevant D. E., Sebbane F., Hinnebusch B. J. (2010). Transit through the flea vector induces a pretransmission innate immunity resistance phenotype in Yersinia pestis. PLoS Pathog 6, e1000783. 10.1371/journal.ppat.1000783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viboud G. I., Bliska J. B. (2005). Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu Rev Microbiol 59, 69–89. 10.1146/annurev.micro.59.030804.121320 [DOI] [PubMed] [Google Scholar]
- Ye Z., Kerschen E. J., Cohen D. A., Kaplan A. M., van Rooijen N., Straley S. C. (2009). Gr1+ cells control growth of YopM-negative Yersinia pestis during systemic plague. Infect Immun 79, 3791–3806. 10.1128/IAI.00808-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z., Uittenbogaard A. M., Cohen D. A., Kaplan A. M., Ambati J., Straley S. C. (2011). Distinct CCR2+ Gr1+ cells control growth of the Yersinia pestis ΔyopM mutant in liver and spleen during systemic plague. Infect Immun 79, 674–687. 10.1128/IAI.00808-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.-S., Park C. G., Zhang P., Bartra S. S., Plano G. V., Klena J. D., Skurnik M., Hinnebusch B. J., Chen T. (2008). Plasminogen activator Pla of Yersinia pestis utilizes murine DEC-205 (CD205) as a receptor to promote dissemination. J Biol Chem 283, 31511–31521. 10.1074/jbc.M804646200 [DOI] [PMC free article] [PubMed] [Google Scholar]


