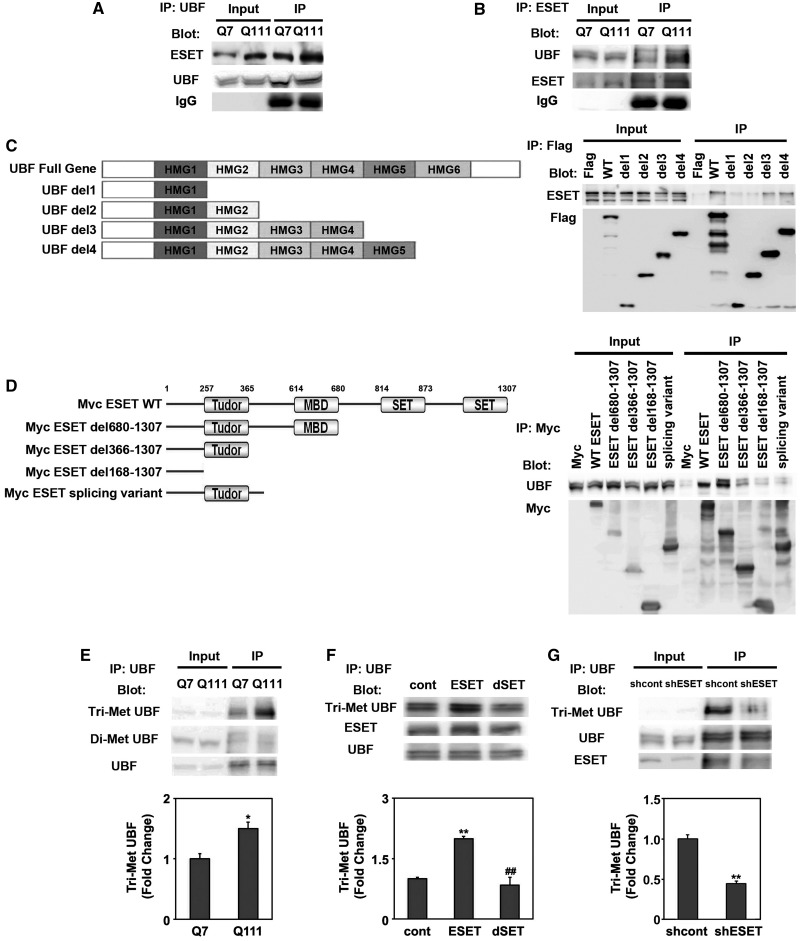
Figure 2.
UBF interacts with ESET and its methylation is modulated by the methyltransferase activity of ESET. (A) UBF interacts with ESET in Q7 and Q111 cells. Cell lysates were immunoprecipitated with anti-UBF antibody and subsequently the blot was probed with anti-ESET antibody. The same blot was stripped and reprobed with anti-UBF antibody. (B) ESET was associated with UBF. (C) A scheme of UBF deletion constructs. Plasmids encoding Flag-UBF and its deletion mutants were transiently transfected in striatal (Q7) cells. The lysates were immunoprecipitated with anti-Flag antibody and the blot was probed with anti-ESET antibody. (D) Schematic representation of ESET functional domains. Plasmids encoding Myc-ESET and its mutants were transfected into Q7 cells. The lysates were immunoprecipitated with anti-Myc antibody and blots were probed with anti-UBF antibody. (E) The level of Tri-Met UBF was increased in Q111 HD cells in comparison with Q7 control cells. The UBF was immunoprecipitated and the blot was probed with anti-Tri-Met-Lys antibody (n = 3). (F) The deletion of SET domain in ESET (ESET–dSET) abrogated the methylation of UBF (n = 3). (G) Knockdown of ESET by shRNA reduced the methylation of UBF (n = 3). ESET (F) and UBF blots (G) were obtained from duplicate gels.