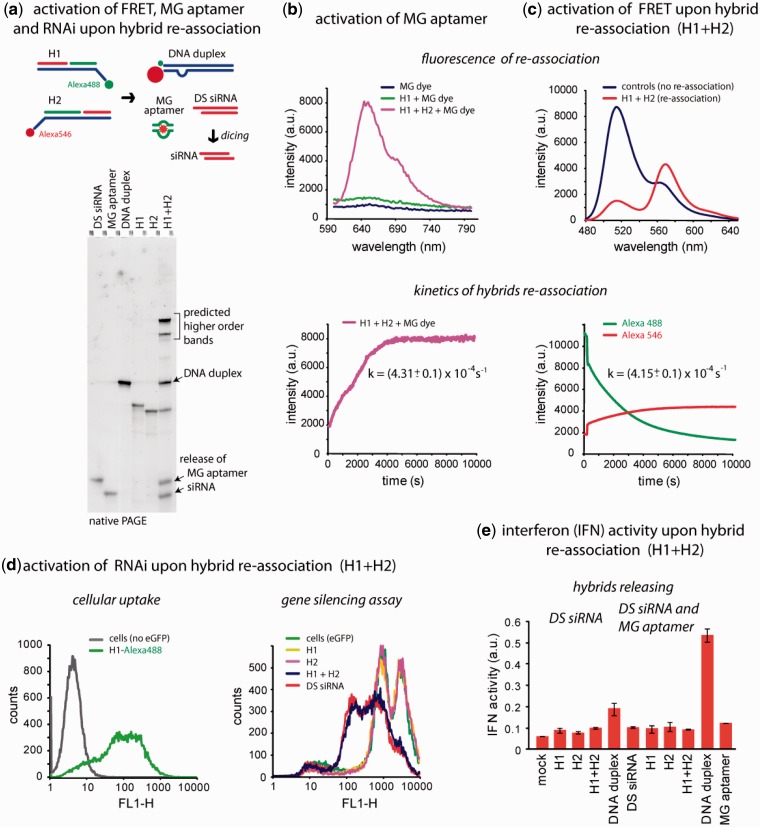
Figure 2.
Release of multiple functionalities (FRET, MG aptamer and DS siRNA) upon re-association of RNA–DNA hybrids. (a) Schematic of hybrid re-association and native PAGE demonstrating the release of DS siRNA and MG aptamer upon re-association of H1(mg1_sDS) and H2(mg2_aDS). Higher order bands on the gel (H1 + H2 lane) are in agreement with the computational predictions and can be attributed to asymmetry of the resulting DNA duplex (36). (b) Static and kinetics fluorescent experiments. Upper panel: activation of MG aptamer during the release. MG by itself is non-fluorescent (blue curve) and the presence of either one of the hybrids does not activate its fluorescence (green curve). However, re-association of two cognate hybrids leads to the release of individual MG aptamer strands, their assembly and further MG uptake leading to the significant increase of its fluorescence (magenta curve). Lower panel: kinetics time trace of the MG aptamer formation during hybrid re-association. (c) FRET activation. Upper panel: activation of FRET during re-association. Emission spectra of control DNA duplexes showing no FRET (blue curve) and re-associated hybrids with increased Alexa546 emission signal (red curve). Lower panel: FRET time traces during re-association of hybrids labeled with Alexa488 and Alexa546. (d) Cell culture experiments. Upper panel: cellular uptake of the fluorescently labeled hybrids. Lower panel: GFP knockdown assays. Three days after the transfection of cells, eGFP expression was statistically analyzed with flow cytometry experiments. As the control, DS siRNA duplexes against eGFP were used. (e) IFN activity was assessed using THP-1 IFN reporter cells that secrete alkaline phosphatase in response to type I IFN. Cells were transfected with hybrids, and culture supernatants were assayed for reporter activity after 24 h.