Figure 5.
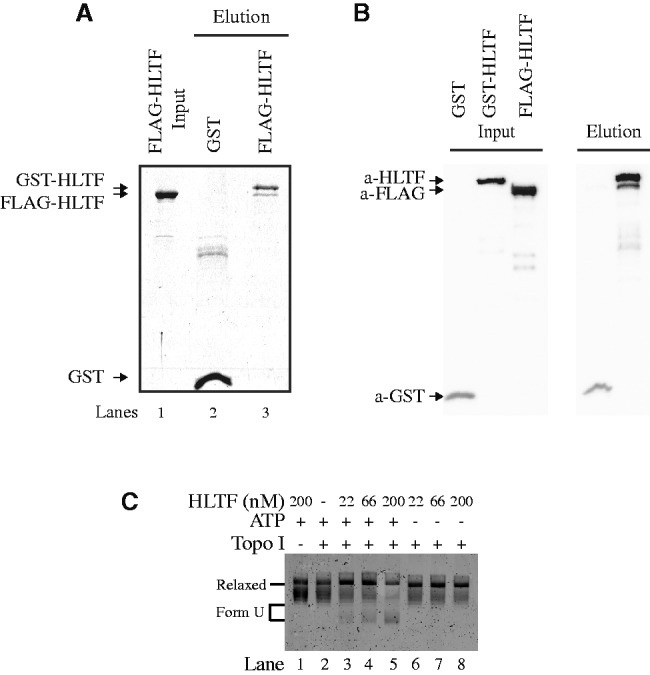
Mechanism of D-loop formation by HLTF. (A) Self-association of HLTF. GST pull-down experiment was carried out using FLAG-HLTF and GST-HLTF or GST followed by immobilization on GTH beads. The beads were washed, bound proteins were eluted and reactions were analysed on 10% SDS–PAGE gels. (B) Western blot analyses of the GST pull-down experiment. We sequentially developed the filter by anti-FLAG, anti-GST and finally anti-HLTF antibodies as indicated on the bottom of the gels together with the bands corresponding to individual proteins. (C) HLTF mediates change in DNA conformation in ATP-dependent manner. Increasing amounts of HLTF (22, 66 and 200 nM) were incubated with topologically relaxed DNA and topoisomerase I with (lanes 2–5) or without (lanes 6–8) ATP as indicated. Lane 1 represents control reaction in the absence of topoisomerase I. All reactions were stopped by addition of SDS/proteinase K and reaction products resolved on 0.8% native agarose gel. Position of Relaxed and Form U DNA is indicated.
