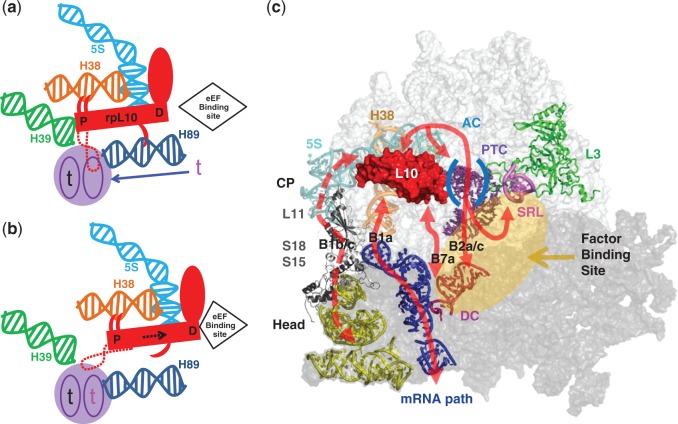
Figure 6.
Models of rpL10 function: rpL10 is at the center of a cascade of allosteric communication pathways throughout the ribosome. (a) The rpL10 loop ‘flipped in’ conformation is the substrate for aa-tRNA and Sdo1p. P, PTC-proximal; D, PTC-distal. (b) The ‘flipped out’ loop conformation, substrate for eEF2. Binding of an aa-tRNA (indicated by the red ‘t’) causes displacement of the loop from the A-site, precipitating structural rearrangements in rpL10. These include lateral displacement of the main body of the protein (dashed black arrow) and H38 toward the elongation factor binding site, creating the binding platform for eEF2. Release of the N-terminal hook of rpL10 from H89 enables closing of the aa-tRNA AC. These movements also initiate allosteric transmission of information through the communication pathways shown in (c) to distantly located functional centers of the ribosome to set the stage for the next phase of elongation. These include rearrangements in the E-site in preparation for release of deacylated tRNA, and interactions with the decoding center and SSU to initiate subunit rotation. (c) Summary of chemical probing experiments mapping the allosteric information exchange pathways emanating from rpL10 to all the functional centers of the ribosome to influence intersubunit rotation. Intersubunit bridges B1a, B1b/c B2a/c and B7a and ribosomal proteins L3, L10, L11, S15 and L18 are labeled. CP, central protuberance of the LSU; SRL, Sarcin/Ricin loop; DC, decoding center.