Abstract
The prevalence of gastric ulcers is high in cholestatic patients, but the exact mechanism of this increased frequency remains uncertain. It has been shown that pioglitazone accelerates the healing of pre-existing gastric ulcers. The present study was designed to investigate the effect of pioglitazone, on the gastric mucosal lesions in cholestatic rats. Cholestasis was induced by surgical ligation of common bile duct and sham-operated rats served as control. Different groups of sham and cholestatic animals received solvent or pioglitazone (5, 15, 30 mg/kg) for 7 days. On the day eight rats were killed after oral ethanol administration and the area of gastric lesions was measured. The serums of rats were also collected to determine serum levels of tumour necrosis factor alpha (TNF-α), IL-1β and bilirubin. The ethanol-induced gastric mucosal damage was significantly more severe in cholestatic rats than sham-operated ones. Pretreatment with pioglitazone dose-dependently attenuated gastric lesions induced by ethanol in both sham and cholestatic rats, but this effect was more prominent in cholestatic ones. The effect of pioglitazone was associated with a significant fall in serum levels of TNF-α in cholestatic rats. L-NAME, a non-selective nitric oxide synthase (NOS) inhibitor, and decreased pioglitazone-induced gastroprotective effect in cholestatic rats, while aminoguanidine, a selective inducible NOS inhibitor, potentiated pioglitazone-induced gastroprotective effect in the cholestatic rats. Chronic treatment with pioglitazone exerts an enhanced gastroprotective effect on the stomach ulcers of cholestatic rats compared to sham rats probably due to constitutive NOS induction and/or inducible NOS inhibition and attenuating release of TNF-α.
Keywords: cholestasis, gastric ulcers, nitric oxide, pioglitazone, rat, tumour necrosis factor alpha
The frequency of gastrointestinal ulceration is higher in jaundiced patients than in the normal population (Bastid et al. 1990). Experimental studies have shown that the gastric mucosa of cholestatic animals is more vulnerable to stress (Sasaki et al. 1986) and to gastroinvasive agents such as aspirin and bile salts than the gastric mucosa of intact animals (Matsuo et al. 1989; Dehpour et al. 1998). The exact mechanism of this increased frequency still remains uncertain, but the most important theory is that it is caused by a decrease in gastric wall blood flow (Sasaki et al. 1986; Urakawa et al. 1987). Previous reports have also referred to increased gastric acid output (Sasaki et al. 1986), decreased mucosal noradrenaline and prostaglandin E2 (Urakawa et al. 1987), and increased free radical formation (Ito et al. 1993; Shian et al. 1994) in rats with obstructive cholestasis. On the other hand, several studies reported that there is overproduction of nitric oxide (Geraldo et al. 1996; Ghafourifar et al. 1997; Inan et al. 1997) and proinflammatory cytokines (Assimakopoulos et al. 2007) in cholestasis.
The peroxisome proliferator-activated receptor γ (PPARγ) is a member of the nuclear receptor superfamily of ligand-dependent transcription factors that is predominantly expressed in adipose tissue, where it has been shown to have a key role in adipogenesis (Kliewer et al. 1994; Tontonoz et al. 1995). Activated PPAR forms a heterodimer with retinoid X receptor and alters the transcription of numerous target genes after binding to specific peroxisome proliferator response elements (PPREs), consisting of a hexameric direct repeat (TGACCT) separated by a single nucleotide (Kliewer et al. 1992; Forman et al. 1995). Additional functions such as regulation of inflammation, control of cell cycle and apoptosis were attributed to PPAR, suggesting a more pleiotropic role in multiple fundamental pathways with wide-ranging biomedical implications (Rocchi & Auwerx 1999; Everett et al. 2000).
PPARg is predominantly expressed in the adipose tissue and colon, whereas stomach, small intestine, liver and pancreas express lower but significant levels (Braissant et al. 1996). The broad pattern of its distribution indicates its possible involvement in multiple biological processes. Konturek et al. (2003a,b) for the first time showed that administration of pioglitazone was associated with a dose-dependent acceleration of ulcer healing in acetic-acid induced ulcers of rats and this was accompanied by a significant increase in the gastric blood flow at the ulcer margin. Konturek et al. (2003a,b) also showed that pioglitazone reduces the acute erosions and deeper gastric lesions induced by ischaemia/reperfusion in rat. The gastroprotective and hyperaemic actions of pioglitazone on the stomach was attributed to endogenous nitric oxide and prostaglandin and attenuation of the expression and release of proinflammatory cytokines tumour necrosis factor alpha (TNF-α) and interleukin 1 (IL-1). It has also been shown that pioglitazone accelerates ulcer healing, possibly due to the enhancement in angiogenesis at ulcer margin (Brzozowski et al. 2005).
The present study was designed to investigate the effect of pioglitazone on gastric mucosal lesions of cholestatic rats induced by ethanol. We also tried to clarify the role of nitric oxide synthase (NOS) isoenzymes such as constitutive NOS (cNOS) and inducible NOS (iNOS) and of proinflammatory cytokines such as IL-1β and TNF-α in the effects of pioglitazone.
Materials and methods
Ethical approval
The protocol for the study was approved by the Ethics Committee of the Shiraz University of Medical Sciences, and all experiments were performed according to the institutional guidelines for animal care and use. Animals were kept under standard conditions with standard rat chow and water ad libitum.
Chemicals
These chemicals were used: pioglitazone (Sigma, St. Louis, MO, USA), NG-nitro-L-arginine methyl ester (L-NAME), a non selective inhibitor of NOS (Sigma), aminoguanidine, a selective inhibitor of inducible NOS (Sigma-Aldrich, St. Louis, MO, USA), ethanol (Merck, Darmstadt, Germany), Ketamine (Rotex medica, Trittau, Germany), Xylazine (Alfasan, Woerden, Holland). Pioglitazone was suspended in 0.5% carboxy methyl cellulose. The pioglitazone suspension and ethanol were administered orally by gavage. Other agents including L-NAME and aminoguanidine were dissolved in saline, and administered intraperitoneally. All drugs were prepared immediately before use.
Experimental design and protocol
Male Sprauge-Dawley rats, weighing 200–250 g were randomly divided into 16 groups; 6–7 rats each. Experiments were performed in two sets comprising eight groups of sham rats and eight groups of cholestatic rats. Animals were anaesthetized by a single intraperitoneal injection of Ketamine (50 mg/kg) and Xylazine (10 mg/kg). Midline laparotomy was performed, and bile duct was isolated and doubly ligated using Cameron and Oakley method (Cameron & Oakley 1932). Sham-operated animals were subjected to laparotomy, bile duct identification and manipulation without ligation. Six days after the operation, the rats were fasted for 24 h but were allowed free access to water. During this period, the animals were housed in individual cages with wire net floor to prevent coprophagy.
Four groups of sham rats were given vehicle (normal saline) or pioglitazone at doses of 5, 15 or 30 mg/kg/day. Four groups of cholestatic rats were treated similarly. Four groups of sham rats were given L-NAME (10 mg/kg), aminoguanidine (100 mg/kg), L-NAME (10 mg/kg) + pioglitazone (5 mg/kg), or aminoguanidine (100 mg/kg) + pioglitazone (5 mg/kg). Four groups of cholestatic rats were treated similarly.
On day 8, animals were given one ml ethanol (96%; Gyires 1994), and were anesthetized with Ketamine and Xylazine 1 h later. Then a midline laparotomy was performed, and blood samples were obtained from abdominal aorta. The stomachs of the animals were removed, and injected with 10 ml formalin (2%) to fix the inner layers of the gastric wall. After 20 min, incision was made along the greater curvature of the stomachs (Hara et al. 1991). The area of the erosions in mm2 was calculated by measuring the width and length of erosions under a stereoscopic microscope. Mucosal lesions were measured using Autocad software 2010.
Blood samples were allowed to clot for 1 h in room temperature, and serums were separated by centrifuging the samples at 1000 g for 20 min. The serums were kept frozen at −80 until analysis. Serum levels of TNF-α and IL1-β were determined using Rat TNF-α/TNFSF1A Immunoassay kit (R&D Systems, Inc., Minneapolis, MN, USA) and Rat IL-1 β/IL-1F2 Immunoassay kit (R&D Systems, Inc.), respectively. Bilirubin was determined with a commercially available kit (Zistshimi, Tehran, Iran) and serum total protein level was determined by spectrophotometry at 546 nm (Pars Azmoon, Tehran, Iran).
Statistics
Data, presented as mean ± SEM, were analyzed using student t test, One Way Analysis of Variance or Two Way Analysis of Variance. Where a significant difference was located with anova, the source of difference was located using Tukey test. A P value of ≤0.05 was considered statistically significant.
Results
Induction of cholestasis
Two days after bile duct ligation, the animals showed signs of cholestasis including jaundice, dark urine and steatorrhea. On day 7, after the ligation, total serum bilirubin (4.9 ± 0.5 μM in sham-operated group vs. 89 ± 11.3 μM in cholestatic group) was significantly (P < 0.01) higher that of sham rats. None of the cholestatic rats showed signs of ascites at day 7 postligation. The manifestations of cholestasis were not seen in any of the sham-operated animals.
Gastric mucosal damage in cholestatic rats
As it has been shown in Figure 1, gastric mucosal damage was significantly (P < 0.01) more severe in cholestatic rats (219.8 ± 38.99 mm2) than in sham ones (106 ± 9.3 mm2).
Figure 1.
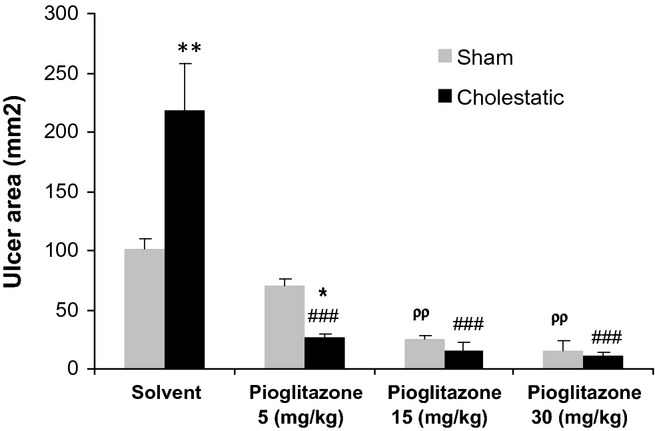
Effect of chronic treatment with solvent or pioglitazone (5, 15, 30 mg/kg) on ethanol-induced gastric ulcers in cholestatic and sham animals. Six to seven rats were used in each group. Data values are expressed as means ± SEM. *P < 0.05 and **P < 0.01 in comparison with the corresponding sham group, ###P < 0.001 in comparison with the cholestatic group received solvent and ρρP < 0.01 in comparison with the sham group received solvent.
Effects of pioglitazone on gastric mucosal damage of cholestatic and sham rats
Pioglitazone administration at 5, 15 or 30 mg/kg was associated with a significant decrease in gastric mucosal lesions in cholestatic rats compared with solvent (P < 0.001). Chronic administration of pioglitazone (5, 15 and 30 mg/kg) also decreased the severity of ethanol-induced gastric damage in sham rats compared with solvent (P < 0.01).
As has been shown in Figure 1, the attenuating effect of pioglitazone on gastric ulcers was more prominent in cholestatic rats because without pioglitazone pretreatment, there was a significant difference between gastric ulcers of sham and cholestatic groups (P < 0.01); after chronic treatment with pioglitazone (10 and 15 mg/kg) there was no difference between gastric ulcers of sham and cholestatic groups. Besides that, the cholestatic-pioglitazone (5 mg/kg) group has decreased gastric ulcers compared to the sham-pioglitazone (5 mg/kg) one, which means that pioglitazone acts as a more potent gastroprotective agent in cholestatic rats.
The role of nitric oxide in the gastroprotective effect of pioglitazone
Chronic co-administration of pioglitazone (5 mg/kg) and L-NAME (10 mg/kg), a non-selective inhibitor of NOS, in cholestatic animals, decreased pioglitazone-induced gastroprotective effects compared with cholestatic rats that received only pioglitazone (5 mg/kg; P < 0.05), while in sham rats L-NAME (10 mg/kg) did not change the gastric healing effect of pioglitazone (5 mg/kg; Figure 2).
Figure 2.
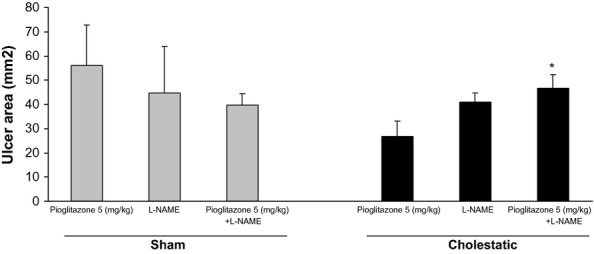
Effect of pioglitazone (5 mg/kg), L-NAME (10 mg/kg) and co-administration of pioglitazone (5 mg/kg) with L-NAME (10 mg/kg) on ethanol-induced gastric ulcers in sham and cholestatic rats. There were six to seven rats in each group. Data are shown as means ± SEM. *P < 0.05 in comparison with the cholestatic group, which received only pioglitazone (5 mg/kg).
Simultaneous chronic treatment of cholestatic rats with pioglitazone (5 mg/kg) along with aminoguanidine, a selective inhibitor of iNOS (100 mg/kg), increased pioglitazone-induced gastroprotective effect compared to cholestatic rats that received only pioglitazone (5 mg/kg; P < 0.05). Chronic treatment of sham rats with pioglitazone (5 mg/kg) along with aminoguanidine (150 mg/kg), did not change the gastric healing effect of pioglitazone alone (5 mg/kg; Figure 3).
Figure 3.
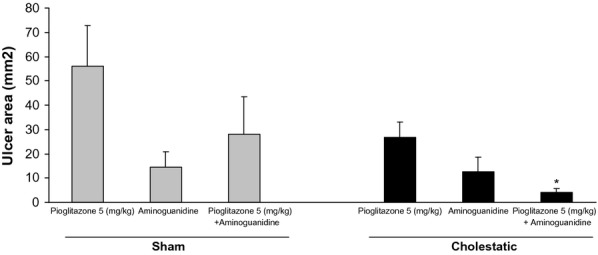
Effect of pioglitazone (5 mg/kg), aminoguanidine (100 mg/kg) and co-administration of pioglitazone (5 mg/kg) with aminoguanidine (100 mg/kg) on ethanol-induced gastric ulcers in sham and cholestatic rats. There were six to seven rats in each group. Data are shown as means ± SEM. *P < 0.05 in comparison with the cholestatic group, which received only pioglitazone (5 mg/kg).
Serum concentration of tumour necrosis factor alpha and IL-1β
Serums TNF-α levels have been shown in Figure 4. Serum TNF-α levels are significantly higher in cholestatic rats compared with sham ones (P < 0.05). Moreover, chronic treatment with pioglitazone at 30 mg/kg was associated with a significant fall in serum levels of TNF-α in cholestatic rats (P < 0.05). Chronic treatment with different doses of pioglitazone did not change TNF-α serum concentration in sham rats.
Figure 4.
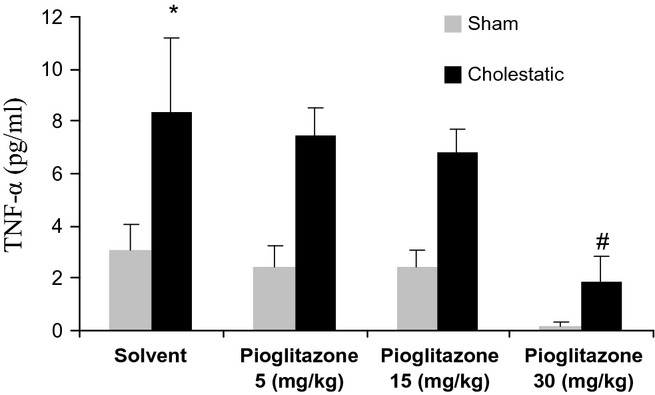
Comparison of serum levels of TNF-α between cholestatic and sham-operated rats given solvent or pioglitazone (5, 15, 30 mg/kg), chronically for 7 days. There were six to seven rats in each group. Data are shown as means ± SEM. *P < 0.05 in comparison with the sham group, which only received solvent, and #P < 0.05 in comparison with the cholestatic group, which only received solvent.
Serum concentration of IL-1β was not significantly different between cholestatic and sham rats. Moreover, chronic treatment with different doses of pioglitazone did not change IL-1β serum concentrations in cholestatic or sham rats (Figure 5).
Figure 5.
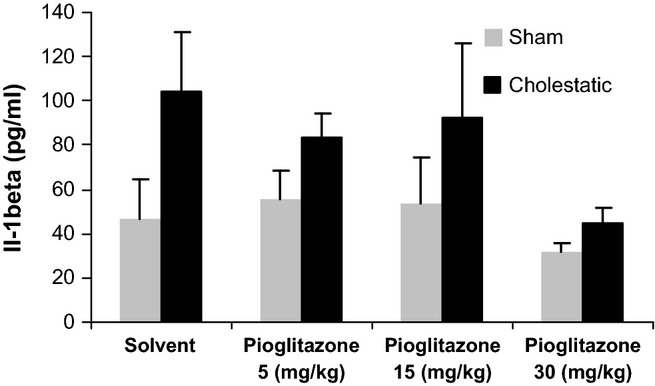
Comparison of serum levels of IL-1β between cholestatic and sham-operated rats given solvent or pioglitazone (5, 15, 30 mg/kg), chronically for 7 days. There were six to sevenrats in each group. Data are shown as means ± SEM.
Serum concentration of total protein
We measured total protein levels in the serum of sham and cholestatic groups treated with solvent or different doses of pioglitazone. There was no difference between the total proteins of different groups 7 days after bile duct ligation (Figure 6).
Figure 6.
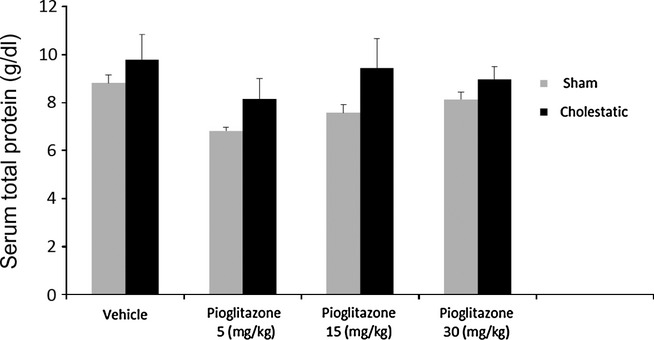
Serum total protein levels in cholestatic or sham rats on the seventh day after the surgery. There were six to seven rats in each group. Data are shown as means ± SEM.
Discussion
It is well known that fatal upper gastrointestinal bleeding often occurs in critically ill or postoperative patients with obstructive jaundice (Urakawa et al. 1987) and the frequency of gastrointestinal ulcerations are higher in jaundiced patients compared with normal population (Bastid et al. 1990). Several experimental studies have shown that the gastric mucosa of cholestatic animals is more vulnerable to water-immersion stress (Sasaki et al. 1986) and gastroinvasive agents such as aspirin, indomethacin, ethanol and taurocholate (Matsuo et al. 1989; Dehpour et al. 1998, 1999; Nahavandi et al. 2001). Our results in this study also are consistent with previous reports. We showed that ethanol-induced gastric mucosal damage was significantly more severe in cholestatic rats than in sham-operated ones. In our previous study which was performed in cirrhotic rats (28 days after bile duct ligation), we also indicates that the gastric mucosal ulcers were significantly more severe in cirrhotic animals than in sham ones, which indicates that the gastric susceptibility to damage persists for several days (Moezi et al. 2013).
The first report about the beneficial effect of PPAR-γ ligand on healing of chronic gastric ulcer was from Konturek et al. (2003a,b). They showed that PPAR-γ was expressed at the margin of the gastric ulcers and that pioglitazone dose-dependently accelerated the ulcer healing in acetic-acid induced ulcers of rats, the effect being accompanied by increased gastric mucosal blood flow at the ulcer margin. Their observation was supported by the fact that the mRNA expression for PPAR-γ was significantly upregulated at the ulcer edge compared to the intact gastric mucosa (Konturek et al. 2003a,b). The gastroprotective effect of pioglitazone was also hinted in other studies (Konturek et al. 2003a,b; Brzozowski et al. 2005; Lahiri et al. 2009). In this study, we showed that pretreatment of rats with pioglitazone (5, 15 and 30 mg/kg) attenuated gastric lesions induced by ethanol in sham rats, which is in line with previous studies. Besides that, for the first time, we showed that chronic treatment with different doses of pioglitazone (5, 15 and 30 mg/kg) decreased ethanol-induced gastric lesions in cholestatic rats too. Interestingly, we indicated that the attenuating effect of pioglitazone on gastric ulcers was more intensive in cholestatic rats. The reason for this conclusion is that while there were many more gastric ulcers in the cholestatic-solvent group compared to the sham-solvent one, the cholestatic-pioglitazone (5 mg/kg) group has decreased numbers of gastric ulcers compared to the sham-pioglitazone (5 mg/kg), which implies that pioglitazone acts as a more potent gastroprotective agent in cholestatic rats. Besides that, after chronic treatment with pioglitazone (10 and 15 mg/kg), there was no difference between the gastric ulcers of sham and cholestatic groups. These data are in line with our previous report about cirrhotic animals which shows the same results (Moezi et al. 2013).
One possible mechanism in gastroprotective activity of pioglitazone in cholestasis could be the modulatory effect of nitric oxide in the pioglitazone effects.
Nitric oxide has an important role in the regulation of gastric wall blood flow (Pique et al. 1989) and gastric acid and mucus secretion (Martinez-Cuesta et al. 1992; Brown et al. 1993). Both NOSs (constitutive and inducible) have been detected in gastric mucosal cells isolated from rats (Nishida et al. 1997). In the digestive system, nitric oxide produced by constitutive NOS is assumed to be cytoprotective, while excessive nitric oxide produced by iNOS is cytotoxic (Nishida et al. 1997). Several studies have also suggested that there is overproduction of nitric oxide in experimental models of bile duct obstruction (Geraldo et al. 1996; Ghafourifar et al. 1997; Inan et al. 1997). We have suggested an important role for nitric oxide in the pathophysiology of peptic ulcers in cholestatic animals. Nitric oxide can potentiate development of gastric mucosal damage in cholestatic subjects. Nitric oxide synthesis inhibition significantly enhances the development of gastric mucosal lesions in control rats, while in cholestatic animals, it decreases severity of gastric damage (Nahavandi et al. 1999). This effect has been explained by overproduction of nitric oxide in cholestasis, which is associated with oxidative stress, cell death and gastrointestinal injury (Nishida et al. 1997).
Based upon the previous studies, the contribution of nitric oxide has been revealed in different functions of thiazolidinediones (Kitamura et al. 1999; Heneka et al. 2000; Allami et al. 2011). Since 2000, when Heneka et al., suggested the therapeutic role of PPARγ agonist in Alzheimer's disease by preventing iNOS expression and neuronal cell death (McIntyre et al. 2006), many other studies have focused on the role of nitric oxide in the beneficial effects of thiazolidinediones in different disorders including Alzheimer's disease (Heneka et al. 2005; Sundararajan et al. 2005), stroke (Pereira et al. 2006), Parkinson's disease (Breidert et al. 2002; Schintu et al. 2009), and multiple sclerosis (Feinstein et al. 2002). In the cardiovascular system, PPARγ activation increases the bioavailability of nitric oxide (Bagi et al. 2004). Peroxisome proliferator-activated receptor γ analogues significantly increases nitric oxide release from pulmonary endothelial cells and enhances calcium-dependent nitric oxide release from umbilical vein endothelial. Previous studies also reported that PPAR-γ ligands inhibit the expression of iNOS and gelatinase B, in part by antagonizing the activities of transcription factors such as NFκB, which have been implicated in the mechanism of ulcer healing (Ricote et al. 1998; Takahashi et al. 2001). Konturek et al. (2003a,b), has demonstrated that in normal rats, pioglitazone dose-dependently inhibited gastric mRNA expression of iNOS, which is accompanied by a compensatory increase in cNOS expression.
According to our experiments, co-administration of L-NAME, a non-selective inhibitor of NOS, with pioglitazone increased gastric ulcers compared with pioglitazone alone. This finding showed that L-NAME reduced the gastroprotective effect of pioglitazone in cholestatic rats. On the other hand, co-administration of pioglitazone and aminoguanidine, a selective inhibitor of iNOS, reduced gastric mucosal lesions in cholestatic rats compared with cholestatic animals that received pioglitazone alone; in the other words, co-administration of pioglitazone and aminoguanidine potentiates pioglitazone-induced gastroprotective effect in cholestatic rats. On the basis of these two findings, the possible mechanism of pioglitazone in gastric ulcers of cholestatic rats might be inducible NOS inhibition or constitutive NOS induction. L-NAME which blocks both constitutive NOS and inducible NOS during pioglitazone treatment reduced gastroprotective effect of pioglitazone in cholestatic rats, while aminoguanidine, which blocks only inducible NOS, enhanced the gastroprotective effect of pioglitazone. From these results, we can conclude that pioglitazone might inhibit inducible NOS and/or induce constitutive NOS. There are several reports about the gastroprotective effect of constitutive NOS including the report of Ma & Wallace (2000) who demonstrated that nitric oxide derived from iNOS does not play an important role in gastric ulcer healing. Although they could not exclude a role for neuronal NOS-derived nitric oxide, their data suggested that eNOS-derived nitric oxide is most important in terms of effects on the healing process, most likely through its effects on angiogenesis (Ma & Wallace 2000). Akiba et al. (1998) demonstrated that inhibition of inducible NOS delayed gastric ulcer healing in rats. The same results were attained in cirrhotic rats, which showed the possible constitutive NOS induction or inducible NOS inhibition by pioglitazone (Moezi et al. 2013).
According to our results in sham rats, L-NAME (10 mg/kg) did not change the gastric healing effect of pioglitazone, while Brzozowski et al. (2005) reported that L-NNA (10 mg/kg), another non-selective NOS inhibitor, attenuated the protective and hyperaemic activity of pioglitazone in control animals. This difference between results might be because of differences in the dose and type of drugs which have been used. Chronic treatment of sham rats with pioglitazone (5 mg/kg) along with aminoguanidine also did not change the gastric healing effect of pioglitazone. The difference in data of chronic treatment of pioglitazone between sham and cholestatic rats might be dependent on overproduction of nitric oxide in cholestasis which has been indicated in several previous papers.
The key event in the pathophysiology of obstructive jaundice-associated complications is endotoxaemia of gut origin because of intestinal barrier failure (Assimakopoulos et al. 2007). The excessive presence of endotoxin in the portal and systemic circulation stimulates a systemic inflammatory response characterized by the release of cytokines and other proinflammatory cytokines such as TNF-α, (IL-1), IL-1, IL-6, interferon–gamma (INF–γ), nitric oxide and oxygen free radicals (Assimakopoulos et al. 2007). In the current study, cholestatic rats had increased level of serum TNF-α level. There was also a trend of higher serum IL1-β level in the cholestatic group which did not reach statistical significance.
Konturek et al. (2003a,b) reported that pioglitazone accelerates ulcer healing via the mechanism involving the decrease in expression and release of TNF-α and interleukin-1β. Our experiments have shown that pretreatment with pioglitazone (30 mg/kg) was associated with a significant fall in serum levels of TNF-α in cholestatic rats. There was also a lower serum IL1-β level in the cholestatic group which received pioglitazone (30 mg/kg) but this was not statistically significant. In our previous paper, we demonstrated that suppression of IL1-β, not TNF-α, could be a mechanism in pioglitazone-induced healing effect of gastric ulcers in cirrhotic rats (Moezi et al. 2013). Although several reports demonstrated that cytokines including TNF-α and IL1-β are increased in bile duct ligation, there are also some reports with conflicting results. For example, Plebani et al. (1999) found a fluctuation pattern for TNF-α in bile duct-ligated rats. They indicated that high values of TNF-α were recorded soon after surgery of bile duct ligation, followed by a decrease on the first and second days and then by two peaks on day 8 and 14 (Plebani et al. 1999). Therefore, the differences between the results of our previous study in cirrhosis and our present results in cholestasis about cytokines might be because of fluctuations in basal level of different cytokines during the days after bile duct ligation. More studies need to be done to explore the real mechanism of these differences.
Since cholestasis affects liver function, it might also affect the serum protein levels. This might produce unreal changes in the level of cytokines; in this case, cytokine measurements should be adjusted to the protein contents of the samples. Therefore, we measured total protein levels in the serum of sham and cholestatic groups treated with solvent or different doses of pioglitazone. According to our results, there was no difference between the total proteins of different groups 7 days after bile duct ligation. Therefore, there was no need to adjust cytokine levels to protein contents of the samples. Our results are in line with previous studies including Mun et al. (Plebani et al. 1999) which showed that there was no change in total protein of serum total protein in bile duct-ligated rats.
We conclude that chronic treatment with pioglitazone exerts a potent gastroprotective effect on the stomach ulcers of cholestatic rats probably due to constitutive NOS induction or inducible NOS inhibition and attenuating release of TNF-α.
Acknowledgments
The present article was extracted from the thesis written by Dr Zeinab Janahmadi and was financially supported by Shiraz University of Medical Sciences grants No. 3815-86.
References
- Akiba Y, Nakamura M, Mori M, et al. Inhibition of inducible nitric oxide synthase delays gastric ulcer healing in the rat. J. Clin. Gastroenterol. 1998;27(Suppl 1):S64–S73. doi: 10.1097/00004836-199800001-00011. [DOI] [PubMed] [Google Scholar]
- Allami N, Javadi-Paydar M, Rayatnia F, et al. Suppression of nitric oxide synthesis by L-NAME reverses the beneficial effects of pioglitazone on scopolamine-induced memory impairment in mice. Eur. J. Pharmacol. 2011;650:240–248. doi: 10.1016/j.ejphar.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Assimakopoulos SF, Scopa CD, Vagianos CE. Pathophysiology of increased intestinal permeability in obstructive jaundice. World J. Gastroenterol. 2007;13:6458–6464. doi: 10.3748/wjg.v13.i48.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagi Z, Koller A, Kaley G. PPARgamma activation, by reducing oxidative stress, increases NO bioavailability in coronary arterioles of mice with Type 2 diabetes. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H742–H748. doi: 10.1152/ajpheart.00718.2003. [DOI] [PubMed] [Google Scholar]
- Bastid C, Tellechea J, Sahel J. Does jaundice increase the frequency of gastroduodenal ulcerations? Hepatogastroenterology. 1990;37:612–614. [PubMed] [Google Scholar]
- Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha,-beta, and-gamma in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- Breidert T, Callebert J, Heneka MT, Landreth G, Launay JM, Hirsch EC. Protective action of the peroxisome proliferator-activated receptor-gamma agonist pioglitazone in a mouse model of Parkinson's disease. J. Neurochem. 2002;82:615–624. doi: 10.1046/j.1471-4159.2002.00990.x. [DOI] [PubMed] [Google Scholar]
- Brown JF, Keates AC, Hanson PJ, Whittle BJ. Nitric oxide generators and cGMP stimulate mucus secretion by rat gastric mucosal cells. Am. J. Physiol. 1993;265:G418–G422. doi: 10.1152/ajpgi.1993.265.3.G418. [DOI] [PubMed] [Google Scholar]
- Brzozowski T, Konturek PC, Pajdo R, et al. Agonist of peroxisome proliferator-activated receptor gamma (PPAR-gamma): a new compound with potent gastroprotective and ulcer healing properties. Inflammopharmacology. 2005;13:317–330. doi: 10.1163/156856005774423908. [DOI] [PubMed] [Google Scholar]
- Cameron GR, Oakley CL. Ligation of the common bile duct. J. Pathol. Bacteriol. 1932;35:769–798. [Google Scholar]
- Dehpour AR, Mani AR, Alikhani Z, et al. Enhancement of aspirin induced gastric damage by cholestasis in rats. Fundam. Clin. Pharmacol. 1998;12:442–445. doi: 10.1111/j.1472-8206.1998.tb00969.x. [DOI] [PubMed] [Google Scholar]
- Dehpour AR, Mani AR, Amanlou M, Nahavandi A, Amanpour S, Bahadori M. Naloxone is protective against indomethacin-induced gastric damage in cholestatic rats. J. Gastroenterol. 1999;34:178–181. doi: 10.1007/s005350050240. [DOI] [PubMed] [Google Scholar]
- Everett L, Galli A, Crabb D. The role of hepatic peroxisome proliferator-activated receptors (PPARs) in health and disease. Liver. 2000;20:191–199. doi: 10.1034/j.1600-0676.2000.020003191.x. [DOI] [PubMed] [Google Scholar]
- Feinstein DL, Galea E, Gavrilyuk V, et al. Peroxisome proliferator-activated receptor-gamma agonists prevent experimental autoimmune encephalomyelitis. Ann. Neurol. 2002;51:694–702. doi: 10.1002/ana.10206. [DOI] [PubMed] [Google Scholar]
- Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- Geraldo J, Ferraz P, Wallace JL. Prostaglandins modulate the responsiveness of the gastric microcirculation of sodium nitroprusside in cirrhotic rats. Hepatology. 1996;23:123–129. doi: 10.1002/hep.510230117. [DOI] [PubMed] [Google Scholar]
- Ghafourifar P, Dehpour AR, Akbarloo N. Inhibition by L-NA, a nitric oxide synthase inhibitor, of naloxone-precipitated withdrawal signs in a mouse model of cholestasis. Life Sci. 1997;60:265–270. doi: 10.1016/s0024-3205(97)00115-x. [DOI] [PubMed] [Google Scholar]
- Gyires K. The role of endogenous nitric oxide in the gastroprotective action of morphine. Eur. J. Pharmacol. 1994;255:33–37. doi: 10.1016/0014-2999(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Hara N, Hara Y, Natsume Y, Goto Y. Gastric hyperacidity and mucosal damage caused by hypothermia correlate with increase in GABA concentrations of the rat brain. Eur. J. Pharmacol. 1991;194:77–81. doi: 10.1016/0014-2999(91)90126-b. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Klockgether T, Feinstein DL. Peroxisome proliferator-activated receptor-gamma ligands reduce neuronal inducible nitric oxide synthase expression and cell death in vivo. J. Neurosci. 2000;20:6862–6867. doi: 10.1523/JNEUROSCI.20-18-06862.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Sastre M, Dumitrescu-Ozimek L, et al. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1-42 levels in APPV717I transgenic mice. Brain. 2005;128:1442–1453. doi: 10.1093/brain/awh452. [DOI] [PubMed] [Google Scholar]
- Inan M, Sayek I, Tel BC, Sahin-Erdemli I. Role of endotoxin and nitric oxide in the pathogenesis of renal failure in obstructive jaundice. Br. J. Surg. 1997;84:943–947. doi: 10.1002/bjs.1800840710. [DOI] [PubMed] [Google Scholar]
- Ito H, Asahi H, Horiuchi S. Role of oxygen radicals in the pathogenesis of acute gastric mucosal lesion under obstructive jaundice. Nihon Geka Gakkai Zasshi. 1993;94:225–233. [PubMed] [Google Scholar]
- Kitamura Y, Kakimura J, Matsuoka Y, Nomura Y, Gebicke-Haerter PJ, Taniguchi T. Activators of peroxisome proliferator-activated receptor-gamma (PPARgamma) inhibit inducible nitric oxide synthase expression but increase heme oxygenase-1 expression in rat glial cells. Neurosci. Lett. 1999;262:129–132. doi: 10.1016/s0304-3940(99)00055-5. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Umesono K, Noonan DJ, Heyman RA, Evans RM. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992;358:771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Forman BM, Blumberg B, et al. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc. Natl Acad. Sci. USA. 1994;91:7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konturek PC, Brzozowski T, Kania J, et al. Pioglitazone, a specific ligand of peroxisome proliferator-activated receptor-gamma, accelerates gastric ulcer healing in rat. Eur. J. Pharmacol. 2003a;472:213–220. doi: 10.1016/s0014-2999(03)01932-0. [DOI] [PubMed] [Google Scholar]
- Konturek PC, Brzozowski T, Kania J, et al. Pioglitazone, a specific ligand of the peroxisome proliferator-activated receptor gamma reduces gastric mucosal injury induced by ischaemia/reperfusion in rat. Scand. J. Gastroenterol. 2003b;38:468–476. [PubMed] [Google Scholar]
- Lahiri S, Sen T, Palit G. Involvement of glucocorticoid receptor and peroxisome proliferator activated receptor-gamma in pioglitazone mediated chronic gastric ulcer healing in rats. Eur. J. Pharmacol. 2009;609:118–125. doi: 10.1016/j.ejphar.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Ma L, Wallace JL. Endothelial nitric oxide synthase modulates gastric ulcer healing in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:G341–G346. doi: 10.1152/ajpgi.2000.279.2.G341. [DOI] [PubMed] [Google Scholar]
- Martinez-Cuesta MA, Barrachina MD, Pique JM, Whittle BJ, Esplugues JV. The role of nitric oxide and platelet-activating factor in the inhibition by endotoxin of pentagastrin-stimulated gastric acid secretion. Eur. J. Pharmacol. 1992;218:351–354. doi: 10.1016/0014-2999(92)90191-6. [DOI] [PubMed] [Google Scholar]
- Matsuo T, Sasaki I, Kamiyama Y, et al. Taurocholate-induced gastric damage in rats with obstructive jaundice. Scand. J. Gastroenterol. Suppl. 1989;162:83–86. doi: 10.3109/00365528909091131. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Soczynska JK, Lewis GF, MacQueen GM, Konarski JZ, Kennedy SH. Managing psychiatric disorders with antidiabetic agents: translational research and treatment opportunities. Expert Opin. Pharmacother. 2006;7:1305–1321. doi: 10.1517/14656566.7.10.1305. [DOI] [PubMed] [Google Scholar]
- Moezi L, Heidari R, Amirghofran Z, Nekooeian AA, Monabati A, Dehpour AR. Enhanced anti-ulcer effect of pioglitazone on gastric ulcers in cirrhotic rats: the role of nitric oxide and IL-1beta. Pharmacol. Rep. 2013;65:134–143. doi: 10.1016/s1734-1140(13)70971-x. [DOI] [PubMed] [Google Scholar]
- Nahavandi A, Dehpour AR, Mani AR, Homayounfar H, Abdoli A. N(G)-nitro-L-arginine methylester is protective against ethanol-induced gastric damage in cholestatic rats. Eur. J. Pharmacol. 1999;370:283–286. doi: 10.1016/s0014-2999(99)00168-5. [DOI] [PubMed] [Google Scholar]
- Nahavandi A, Mani AR, Homayounfar H, Akbari MR, Dehpour AR. The role of the interaction between endogenous opioids and nitric oxide in the pathophysiology of ethanol-induced gastric damage in cholestatic rats. Fundam. Clin. Pharmacol. 2001;15:181–187. doi: 10.1046/j.1472-8206.2001.00028.x. [DOI] [PubMed] [Google Scholar]
- Nishida K, Ohta Y, Ishiguro I. Role of gastric mucosal constitutive and inducible nitric oxide synthases in the development of stress-induced gastric mucosal lesions in rats. Biochem. Biophys. Res. Commun. 1997;236:275–279. doi: 10.1006/bbrc.1997.6972. [DOI] [PubMed] [Google Scholar]
- Pereira MP, Hurtado O, Cardenas A, et al. Rosiglitazone and 15-deoxy-Delta12,14-prostaglandin J2 cause potent neuroprotection after experimental stroke through noncompletely overlapping mechanisms. J. Cereb. Blood Flow Metab. 2006;26:218–229. doi: 10.1038/sj.jcbfm.9600182. [DOI] [PubMed] [Google Scholar]
- Pique JM, Whittle BJ, Esplugues JV. The vasodilator role of endogenous nitric oxide in the rat gastric microcirculation. Eur. J. Pharmacol. 1989;174:293–296. doi: 10.1016/0014-2999(89)90324-5. [DOI] [PubMed] [Google Scholar]
- Plebani M, Panozzo MP, Basso D, De Paoli M, Biasin R, Infantolino D. Cytokines and the progression of liver damage in experimental bile duct ligation. Clin. Exp. Pharmacol. Physiol. 1999;26:358–363. doi: 10.1046/j.1440-1681.1999.03042.x. [DOI] [PubMed] [Google Scholar]
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- Rocchi S, Auwerx J. Peroxisome proliferator-activated receptor-gamma: a versatile metabolic regulator. Ann. Med. 1999;31:342–351. doi: 10.3109/07853899908995901. [DOI] [PubMed] [Google Scholar]
- Sasaki I, Miyakawa H, Kameyama J, Kamiyama Y, Sato T. Influence of obstructive jaundice on acute gastric ulcer, intragastric pH and potential difference in rats. Tohoku J. Exp. Med. 1986;150:161–168. doi: 10.1620/tjem.150.161. [DOI] [PubMed] [Google Scholar]
- Schintu N, Frau L, Ibba M, et al. PPAR-gamma-mediated neuroprotection in a chronic mouse model of Parkinson's disease. Eur. J. Neurosci. 2009;29:954–963. doi: 10.1111/j.1460-9568.2009.06657.x. [DOI] [PubMed] [Google Scholar]
- Shian WM, Sasaki I, Kamiyama Y, et al. Mucosal TBA reactants and glutathione levels in acute gastric mucosal injury in rats with obstructive jaundice. Ther. Res. 1994;1:77–83. [Google Scholar]
- Sundararajan S, Gamboa JL, Victor NA, Wanderi EW, Lust WD, Landreth GE. Peroxisome proliferator-activated receptor-gamma ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience. 2005;130:685–696. doi: 10.1016/j.neuroscience.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Fujita T, Yamamoto A. Role of nuclear factor-kappaB in gastric ulcer healing in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G1296–G1304. doi: 10.1152/ajpgi.2001.280.6.G1296. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Spiegelman BM. Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor gamma. Curr. Opin. Genet. Dev. 1995;5:571–576. doi: 10.1016/0959-437x(95)80025-5. [DOI] [PubMed] [Google Scholar]
- Urakawa T, Nagahata Y, Nakamoto M, Kumagai K, Saitoh Y. An approach to the mechanism of acute ulceration in obstructive jaundice. Scand. J. Gastroenterol. 1987;22:634–640. doi: 10.3109/00365528708991911. [DOI] [PubMed] [Google Scholar]


