Figure 1. Crystal structures of Str enolase and human plasminogen.
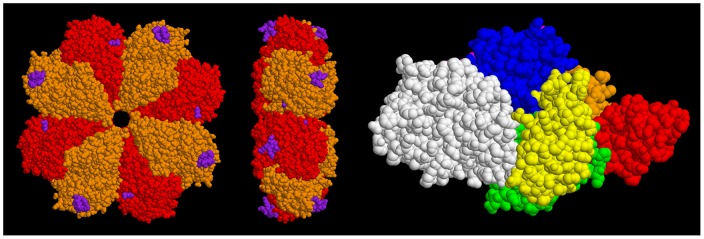
Left figures: Str enolase shown in two views. The homo-octamer is depicted in two colours. The purple atoms represent the sites where Pgn is thought to bind. Right figure: The individual domains of Pgn are colour coded. Residues 1-78 (N-Terminal Peptide), orange. Kringle 1 (79–163), red. Kringle 2 (164–249), green. Kringle 3 (250–345), yellow. Kringle 4 (346–439), blue. Kringle 5 (440–541), magenta. Preproteolytic domain (542–791), white. The figure depicts the closed form whereas it is probable that the open form (for which there is currently no structure) is that which binds to Str enolase. The two proteins are not drawn to the same scale. The dimensions of the Str enolase donut are approximately 15 nm wide and 5 nm thick. The Pgn, in contrast, is not symmetrical and consists of domains attached to, and sticking out from, a continuous string. Its largest dimensions are 10 nm×8.5 nm×5 nm. The dimensions of the two proteins will be important in determining the orientation of the two during the dual polarization interferometry experiments.
