SUMMARY
Purpose:
Up to 30% of patients with idiopathic generalized epilepsy (IGE) have seizures that are refractory to medication despite appropriate therapy that commonly includes valproate (VPA). The aim of this study was to compare patients with VPA-refractory and VPA-responsive IGE in order to determine whether there are group differences in generalized spike and wave discharge (GSWD) generators that may be associated with VPA resistance.
Methods:
Of 89 IGE patients who underwent electroencephalography (EEG) combined with functional magnetic resonance imaging (fMRI; EEG/fMRI), 25 with GSWDs identified in EEG/fMRI data were included. Simultaneous acquisition of 64 channels of EEG data at 10 kHz was performed using an MRI-compatible EEG cap and amplifier at 4T. VPA resistance was defined as lack of seizure control despite therapeutic dose of VPA.
Key Findings:
The fMRI blood oxygen–level dependent (BOLD) correlates of GSWD in the entire group involved midline thalamus, frontal regions comprising Brodmann areas 6, 24, and 32, and temporal lobes diffusely. When VPA-responsive and VPA-resistant patients were compared, BOLD signal increases were noted in the VPA-resistant patients in medial frontal cortex, along the paracingulate gyrus (Montreal Neurological Institute; MNI x = 2, y = 13.6, z = 45.9), and anterior insula bilaterally (right MNI x = 37.6, y = 7.8, z = 0.6, left MNI x = −35.3, y = 13.6, z = −5.3).
Significance:
Our findings support the hypothesis that VPA-resistant and VPA-responsive patients may have different GSWD generators. Furthermore, we hypothesize that these differences in GSWD generators may be the reason for different responses to VPA.
Keywords: Idiopathic generalized epilepsy, Juvenile myoclonic epilepsy, EEG/fMRI, Neuroimaging
Clinically, idiopathic generalized epilepsies (IGEs) are defined as any combination of absence seizures, generalized tonic–clinic seizures, and myoclonus or myoclonic seizures; patients with IGEs usually have electroencephalography (EEG) showing generalized spike and wave discharges (GSWDs) and are cognitively normal. The usual presentation age is preteen or teenage years (Commission, 1989). GSWDs and seizures in IGEs are thought to originate from or involve at some point a bilaterally distributed network of cortical and subcortical areas (Bai et al., 2010; Berg et al., 2010; Szaflarski et al., 2010a). However, the origin of the GSWDs and/or seizures in patients with IGEs remains elusive, with some studies pointing to cortical and others to subcortical (i.e., thalamic) onset (Avoli, 2012; Seneviratne et al., 2012). Previously, several theories regarding the origins of GSWD and seizures in IGEs have been postulated based on animal and human data. These theories include the “centrencephalic theory” put forward by Penfield & Jasper (1954), who proposed that the onset of EEG abnormalities in IGEs is in the midline and intralaminar nuclei of the thalamus; this theory was later redefined by the “thalamic clock” theory based on the examination of cortical and thalamic ablations on high-voltage thalamic spindles (Buzsaki, 1991). The two theories of a peripheral IGE onset include “cortical” and “cortical focus” theories, which are based on the measured delays in GSWD propagation between the hemispheres, and the fact that in some animal models of IGE the leading spikes may originate around the cortical somatosensory areas (Meeren et al., 2005). Finally, the corticoreticular theory proposed by Gloor may be the cement that unifies all the above theories—it suggests that the thalamic and brainstem reticular system is responsible for the genesis of the GSWDs that are a result of or represent abnormal oscillations within the corticoreticular neurons and of interaction between cortical and thalamic neurons (Gloor, 1968). Therefore, based on these theories, the notion of cortical and subcortical involvement in the origination of GSWD in IGEs is evident. However, although all these theories are supported by ample experimental and clinical data, none of them address the question of why some patients respond to antiepileptic drugs (AEDs), including valproate (VPA), which is prototypical for these patients, whereas some patients do not.
Lack of seizure control in IGEs is observed in approximately 10–30% of adult patients taking AEDs (Gelisse et al., 2001; Baykan et al., 2008; Szaflarski et al., 2010b). Some of these patients continue to have seizures despite optimized therapy and management by an epilepsy specialist (Szaflarski et al., 2008). Many features of IGEs are listed as reasons for medication resistance, and include poor adherence to medication regimen, poor sleep hygiene, or other lifestyle factors called globally “pseudo-resistance,” presence of comorbid psychiatric conditions, presence of generalized tonic–clonic seizures, focal EEG features, or early epilepsy onset (Wolf & Inoue, 1984; Fernando-Dongas et al., 2000; Berg et al., 2001; Szaflarski et al., 2008; Iqbal et al., 2009). However, in many patients these or other reasons for poor seizure control cannot be identified. Overall, it has been noted that patients with IGEs tend to respond better to VPA than to other AEDs, and that response to VPA predicts a positive response to another AED (Fernando-Dongas et al., 2000; Szaflarski et al., 2010b). Therefore, VPA is considered the archetypical AED for patients with IGEs with patients’ response to this drug (or lack thereof) possibly identifying different subtypes of IGEs or even differentiating patients with IGEs from patients with other, possibly focal types of epilepsies (e.g., frontal lobe onset based on the “cortical” theory) (Meeren et al., 2005).
Recently, neuroimaging studies have significantly contributed to our understanding of IGEs. Several studies combining EEG with functional magnetic resonance imaging (fMRI; EEG/fMRI) implicated thalamus or specific thalamic nuclei to be involved in GSWD generation more than the cortical regions (Moeller et al., 2008; Tyvaert et al., 2009). Other studies postulated more widespread cortical and subcortical involvement (Aghakhani et al., 2004; Gotman et al., 2005; Hamandi et al., 2006), whereas some studies even suggested that cortical involvement may precede or drive thalamic contribution to the generation of the absence seizures (Bai et al., 2010; Szaflarski et al., 2010a). None of the above studies contradict the theories discussed above, as most if not all of these studies observed interactions between cortical and thalamic activations suggesting again that large cortical and subcortical networks are involved in generation and propagation of GSWD in IGEs. But, none of the above-mentioned studies examined the difference in cortical and subcortical correlates of GSWD generators in patients with VPA-resistant compared to VPA-responsive IGEs. Therefore, in this EEG/fMRI study, we examine the neuroimaging correlates of GSWDs in drug-resistant IGE using EEG/fMRI with VPA as an archetype drug. The central focus of this study is to test the hypothesis that there is a correlation between localization of the blood oxygenation–level dependent (BOLD) signal responses to GSWDs and drug response in patients with IGEs. We hypothesized that in patients with VPA-resistant IGEs, poor seizure control is associated with nonthalamic (possibly cortical) sources of GSWD. By using EEG/fMRI to identify the generators of GSWDs in both groups of IGE patients, we investigated whether there are differences in sources of the GSWDs in patients with VPA-resistant and in VPA-responsive (possibly thalamic) IGEs.
METHODS
Subjects and paradigm
Eighty-nine patients with idiopathic generalized epilepsies (IGEs) were enrolled in the study of spike and wave generators using EEG/fMRI (K23 NS052468); 11 subjects either did not receive EEG/fMRI or did not complete the procedure because of claustrophobia (N = 3), metallic artifact (N = 1), or because of not wanting to continue with the procedure (N = 7). Therefore, data on 78 patients were available for analyses. Diagnosis of IGE was established by an epilepsy specialist based on the criteria put forward by the International League Against Epilepsy (Commission, 1981, 1989). All subjects were recruited from the Cincinnati Epilepsy Center by participating physicians. After confirming that each subject fulfilled all of the inclusion and none of the exclusion criteria, all subjects signed an informed consent form approved by the at the University of Cincinnati Institutional Review Board (IRB) in adherence with the Declaration of Helsinki. For the purpose of this study, response to valproic acid was the outcome variable (Appendix A). In general, we assessed patients as “responsive” (or “good response”) if they became seizure free for at least 3 months while treated with VPA. Patients who did not become seizure free while treated with VPA were assessed as “resistant” (or “poor response”). All other patients were labeled “unknown.” Included in this group were patients who either were never placed on VPA for the treatment of IGE or were placed on this medication but it was subsequently withdrawn because of intolerable side effects with the medication trial lasting <3 months; patients with continued seizures due to lack of medication adherence or because of lack of seizure freedom due to other factors, for example, alcohol use/abuse, poor sleep hygiene, and so on, leading to “pseudo-resistance” were excluded from participation in this study (Szaflarski et al., 2008, 2010b). Expanding this variable to 12 months would be impractical, as many charts do not contain such information (Szaflarski et al., 2008). Specific inclusion/exclusion criteria and detailed criteria for defining good or poor response to VPA are included in Appendix A. Each subject underwent between 1 and 3 consecutive 20-min resting-state EEG/fMRI scans during which they listened to self-selected music with their eyes closed (Difrancesco et al., 2008; Kay et al., 2012). After exclusion of poor-quality scans (N = 10) and scans without GSWDs (N = 131), GSWDs were observed in 37 scans from 25 subjects (see Table 1).
Table 1.
Demographics of subjects from whom generalized spike and wave discharges were recorded
| Valproate | No. scanning runs |
No. male |
No. female |
Ages at scanning | Age at onset | Duration | No. AEDs at scanning |
No. previously failed AEDs |
|---|---|---|---|---|---|---|---|---|
| Resistant | 14 | 3 | 6 | 29.6 ± 15.1 | 11 ± 7.1 | 18.6 ± 19.2 | 2.3 ± 1.0 | 3.9 ± 2.1 |
| Responsive | 12 | 8 | 2 | 29.6 ± 11.6 | 18.5 ± 14.7 | 11.1 ± 5.6 | 1.7 ± 0.5 | 2.7 ± 2.2 |
| Unknown | 11 | 3 | 3 | 27.5 ± 9.0 | 18.8 ± 7.7 | 8.3 ± 8.5 | 1.3 ± 0.5 | 0.8 ± 0.8 |
Number of scanning runs and number of male and female subjects is provided along with mean ± SD of age at scanning age at disease onset, disease duration (years), number of antiepileptic drugs (AEDs) at time of scanning and number of previously failed AEDs
EEG acquisition and processing
EEG acquisition and processing was performed with Scan 4.3.5 software (Compumedics U.S.A., Ltd., El Paso, TX, U.S.A.) as described previously (Espay et al., 2008; Kay et al., 2012). Briefly, subjects were fitted with an MRI-compatible EEG cap with electrodes arranged according to the international 10/20 system, braided carbon cabling, and current-limiting in-line resistors (Compumedics U.S.A., Ltd.). Electrically conductive gel (Quik-Gel; Compumedics Neuromedia Supplies, Charlotte, NC, U.S.A.) was used to establish low impedance (confirmed as <20 k) between each electrode and the scalp. Sixty-four channels of data, including two mastoid channels, one electrocardiography (ECG) channel, and one eye electrooculography (EOG) channel, were recorded at 10 kHz concurrent with fMRI using an MRT-compatible system (MagLink, NeuroScan; Division of Compumedics Ltd., El Paso, TX, U.S.A.). Time marks generated by the scanner were automatically inserted into the data stream at the onset of each volume acquisition.
After completion of the data collection, data were off-line low-pass filtered at 30 Hz, and gradient artifacts were removed using an average artifact subtraction technique (Allen et al., 2000; Difrancesco et al., 2008) based on the time marks inserted by the scanner. A moving average template of five volumes was used to account for changes in the gradient artifact over time. The fidelity of this technique was improved by optimizing the temporal alignment between the average gradient waveform and the raw data using cross-correlation with a shift limit of 25 samples.
Ballistocardiographic (BCG) artifacts were attenuated using a linear spatial filtering technique as described previously (Lagerlund et al., 1997). For each subject, a component of the QRS complex was identified in the ECG channel and used to epoch a majority of heartbeat events in the first functional scan. These epochs were detrended, demeaned, and reviewed for additional artifacts. An average ECG waveform excluding contaminated epochs was obtained. The spatial singular value decomposition (SVD) algorithm in Scan 4.3.5 was used to obtain the principal components of the average waveform. Those components accounting for 99% of signal variance were used to construct a spatial filter in Scan. The filter was applied to EEG data, after gradient artifact removal and decimation to a 1,000 Hz sampling rate, to attenuate the BCG artifact. All processed EEG studies were reviewed by a board-certified epilepsy specialist to identify GSWDs.
MRI acquisition and processing
fMRI was performed on a 4T 61.5 cm bore Varian Unity INOVA system (Varian, Inc., Palo Alto, CA, U.S.A.) equipped with a standard head coil. T2*-weighted, BOLD sensitive, echo-planar images (EPIs) were acquired with an 256 9 256 mm field of view, 64 9 64 voxel matrix, 90-degree flip angle, and 5-mm slice thickness in axial orientation without gap. Four hundred volumes consisting of 30 slices each and repetition time/echo time (TR/TE) = 3,000/2,000 msec were collected during each scan (Difrancesco et al., 2008; Espay et al., 2008). T1-weighted structural images were acquired for use as an anatomic reference. A modified driven equilibrium Fourier transform (MDEFT) method (Duewell et al., 1996; Ugurbil et al., 1999) was used with an 1,100-msec inversion delay, 256 mm 9 196 mm 9 196 mm field of view, 256 9 196 9 196 voxel matrix, 22-degree flip angle, and TR/TE = 13.1/6.0 msec.
Data were reconstructed and corrected for ghosting and geometric distortion with the aid of multiecho reference scans (MERS) (Schmithorst et al., 2001). Functional scans underwent slice-timing correction, motion correction (Jenkinson et al., 2002), rigid-body registration to a high-resolution anatomic scan (Jenkinson & Smith, 2001), non-linear registration (Andersson et al., 2007a,b) to an MNI152 standard, and spatial blurring by a gaussian kernel with full width at half maximum (FWHM) of 6 mm using FMRIB Software Library (FSL) (Smith et al., 2004). The quality of functional to anatomic registration was measured using the mutual information cost function (Jenkinson & Smith, 2001). Functional scans with an outlying cost indicating unsatisfactory registration were excluded from further analysis. The quality of motion correction was measured using the normalized correlation ratio cost function (Jenkinson & Smith, 2001) of each time point to the reference volume. Time points with an outlying cost indicating excessive motion were censored from event-related analysis using the 3dDeconvolve tool in Analysis of Functional NeuroImages (AFNI); the preceding and following time points were also censored (Cox, 1996).
Analysis
The fMRI correlates of GSWDs were examined using an event-related design with the 3dDeconvolve tool in AFNI. The onset times of GSWDs in each scan were obtained from the simultaneous EEG recordings via review by a board-certified epilepsy specialist (JPS). Spike times were convolved with a canonical gamma variate hemodynamic response function given by (t^8.6)*exp(−t/0.547) (Cohen, 1997), which peaks at 4.7 s, and used as the regressor of interest. The six rigid body motion parameters were included as nuisance regressors in the baseline model. T-maps of spike-related fMRI activation were thus obtained for each scan during which GSWDs were observed. The time-evolution of spike-related activation was examined by repeating the preceding analysis for a total of 13 spike-timing shifts. Analysis was repeated with the onset of the hemodynamic response function (HRF) shifted back in time (a shift of –1, –2, …, –6 s), or forward in time (a shift of +1, +2, …, +6 s). A shift of 0 s corresponded to analysis with the original spike timings.
One- and two-sample t-tests were performed at the group level and corrected for multiple comparisons using the 3dmerge tool in AFNI. The mean and standard deviation of positive t-values from group t-maps were obtained for four anatomic regions of interest (ROIs) using the 3dROIstats tool in AFNI. ROIs were taken from the Harvard-Oxford probabilistic cortical and subcortical atlases distributed with FSL using probability >50% (Desikan et al., 2006).
RESULTS
Of the included subjects, 33 were men and 45 were women, age 31.8 ± 11.7 years. Twenty-five of the 25 had clear GSWDs in their EEG/fMRI data and were included in analyses (Table 1). Spike frequency per minute was 4.0 ± 6.4 in VPA-resistant patients, 0.56 ± 0.59 in VPA-responsive patients, and 2.6 ± 4.1 in VPA-unknown patients. The syndromic diagnoses of the analyzed cohort include 12 patients with juvenile myoclonic epilepsy (JME) and 13 with IGEs other than JME (e.g., juvenile absence epilepsy). All patients treated with VPA in the VPA-resistant or VPA-responsive groups had at least one VPA level in a therapeutic range. All patients included in this study are part of a larger study examining the effects of VPA on seizure freedom in patients with IGEs, and data on some of them were included in our previous publications (Szaflarski et al., 2010a,b). Among the included patients, 9 (35%) of the patients with GSWDs were VPA-resistant, 10 (40%) of them were VPA-responsive, and 6 (24%) were VPA-unknown; among patients without GSWDs, 13 (22%) were VPA-resistant, 28 (47%) were VPA-responsive, and 19 (32%) were VPA-unknown. There is no significant difference between all groups (2 9 3 contingency table; p = 0.42), or between VPA-resistant and VPA-responsive patients (p = 0.26).
A two-sided, one-sample t-test was used to identify overall spike-related activation in the entire cohort. Significant (a = 0.05) clusters of spike-related activation (>36 voxels with t > 2.3) are shown in Fig. 1 (left). Diffuse cortical and subcortical activations were observed with strong thalamic activation bilaterally. In general, activation in frontal cortex was observed to occur at earlier offsets of the HRF. Deactivation was observed specifically in regions associated with the default mode network (DMN) at later offsets of the HRF. Affected DMN Brodmann areas (BA) included occipital and parietal BA 19, 29, 31, 7, 19, and 39, and frontal BA 6, 8, 9, 10, and 46.
Figure 1.
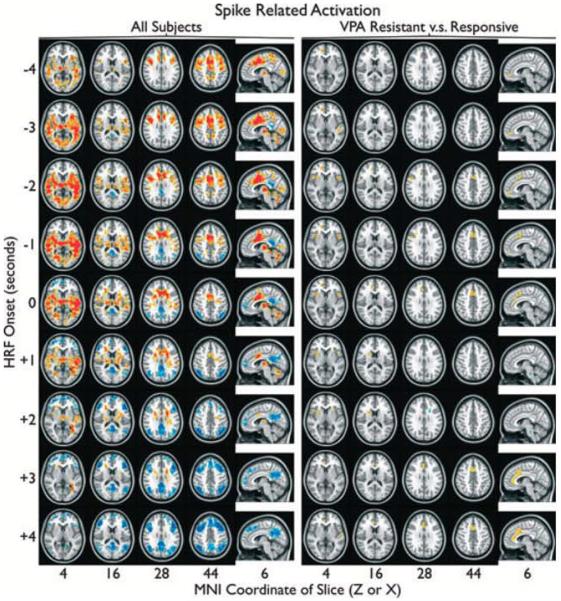
Spike-related activation (red) and deactivation (blue), controlling for number of spikes, are overlayed on the standard MNI anatomic brain in radiologic orientation. Spike times obtained from a simultaneous EEG recording were convolved with a canonical gamma variate hemodynamic response function (HRF)with its peak 4.7 s post onset using AFNI. Activation maps were produced with HRF onset aligied with spikes (0 s), before spikes (−1,−2, … s), and after spikes (+1,+2, … s). Group responses from all subjects (left) and a contrast between vdproate (VPA)-resistant and VPA-responsive subjects(right) were obtained. Significant (a < 0.05) clusters of >36 suprathreshold voxels (left: two-sided t > 2.3, right: one-sided t > 2) are shown.
Epilepsia © ILAE
Next, a one-sided, two-sample t-test was used to contrast spike-related activation between VPA-resistant and VPA-responsive subjects. The number of spikes recorded from each subject was included as a covariate. Significant (a = 0.05) clusters of differences in spike-related activation (>36 voxels with t > 2) are shown in Fig. 1 (right). Increased frontal spike-related activation was observed in VPA-resistant subjects. The affected regions were medial frontal cortex, along the paracingulate gyrus (MNI x = 2, y = 13.6, z = 45.9) and anterior insula bilaterally (right MNI x = 37.6, y = 7.8, z = 0.6, left MNI x = −35.3, y = 13.6, z = −5.3). No significant increase in spike-related activation was observed in VPA-responsive subjects at an HRF offset of 0.
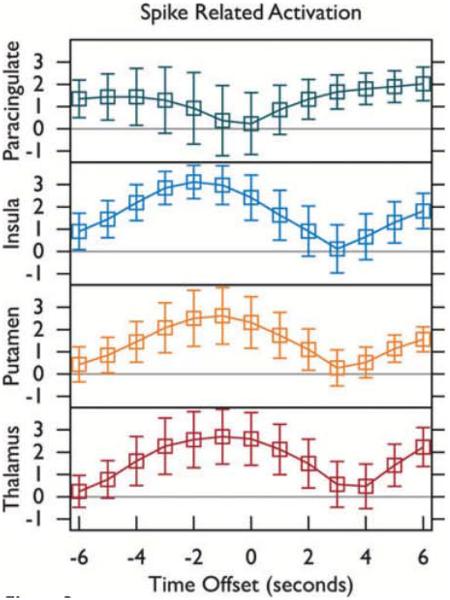
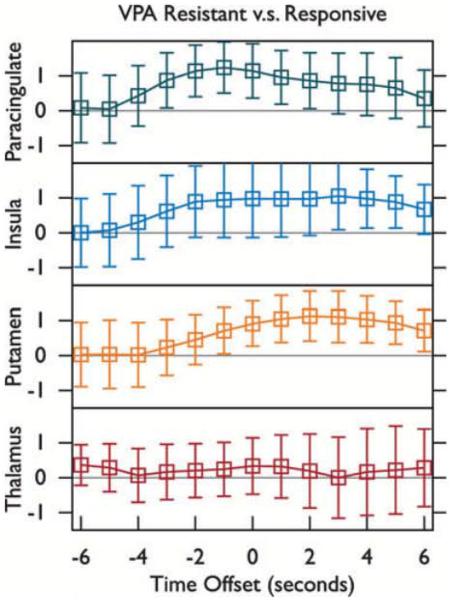
Spike-related activation was further examined in the four anatomic ROIs shown in Fig. 2: thalamus, putamen, insular cortex, and paracingulate (medial frontal) cortex. ROI statistics for overall spike-related activation are shown in Fig. 3. ROI statistics for the contrast in spike-related activation between VPA-resistant and VPA-responsive IGE after controlling for spike count are shown in Fig. 4. The difference between spike-related thalamic activation (Fig. 4, bottom) in VPA-resistant versus VPA-responsive patients did not deviate significantly from zero (a = 0.05), whereas spike-related paracingulate activation (Fig. 4, top) was significantly greater (p < 0.001) in VPA-resistant patients, peaking 1 s before GSWD onset.
Figure 2.
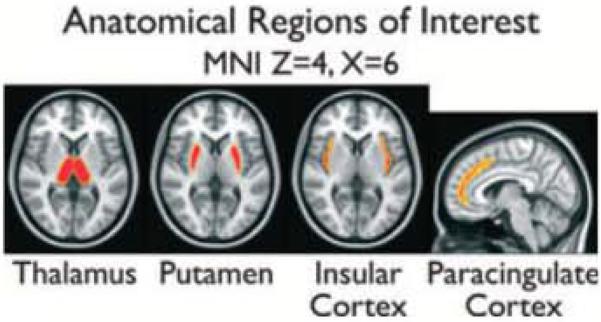
Slices from the anatomic regions of interest (ROIs) used in Figs 3 and 4 are overlayed on the standard MNI anatomic brain In radiologic orientation. Regions were obtained from the Harvard-Oxford cortical and subcortical probabilistic atlases distributed with FSL Voxels with probability >50% of residing within the anatomic region are shown and were used as a mask to compute the ROI time courses in Figures 3 and 4.
Epilepsia © ILAE
Figure 3.
Spike-related fMRI activation was computed at the goup level for all subjects. The average and standard deviation of positive t-values within four regions of interest (ROIs, refer to Fig 2) are shown. spike times obtained from a simultaneous EEG recording were convolved with a canonical gamma variate hemodynamic response function (HRF) with its peak 4.7 s post onset using ARNI. T-maps were produced with HRF onset aligned with spikes (0 s), before spikes (−1,−2, … s), and after spikes(+1,+2, … s).
Epilepsia © ILAE
Figure 4.
A group level contrast for spike-related fMRI activation in valproate (VPA)-resistant versus VPA-responsive subjects was performed. The average and standard deviation of positive t-values within four regions of interest (ROIs, refer to Fig 2) are shown. Spike times obtained from a simultaneous EEG recording were convolved with a canonical gamma variate hemodynamic response function (HRF) with its peak 4.7 s post onset using AFNI. T-maps were produced with HRF onset aligned with spikes (0 s), before spikes (−1, −2, … s), and after spikes (+1,+2, … s).
Epilepsia © ILAE
DISCUSSION
In this EEG/fMRI study, we examined the neuroimaging correlates of GSWDs in patients with IGEs using VPA as an archetype drug. Based on the main theories of the pathophysiology of the generalized epilepsies, we hypothesized that in patients with VPA-resistant IGEs poor seizure control would be associated with cortical sources of GSWDs, whereas VPA-responsive patients would have more typical, thalamic GSWD sources. By using EEG/fMRI, we have shown that all enrolled IGE patients, as a group, have a fairly typical distribution of the BOLD signal changes, whereas clear neuroimaging differences can be observed between patients who do or do not respond to VPA (Fig. 1). In fact, after controlling for the incidence of GSWD in the EEG/fMRI recordings, we were able to show that it is the cortical (but not thalamic) involvement in GSWD generation that differentiates these two groups. Clearly, VPA-resistant patients have much more extensive cortical involvement, which is in agreement with the “cortical” or “cortical focus” theories of IGE pathophysiology (Niedermeyer et al., 1969a; Niedermeyer, 1996; Meeren et al., 2005).
Why is VPA a prototypical AED for patients with IGEs? Although numerous studies have shown that VPA is effective for the control of seizures in patients with IGEs, there is not a single mechanism that is responsible for this effect (Loscher, 2002; Marson et al., 2007; Glauser et al., 2010). In fact, in the experimental models of epilepsy, the anticonvulsant effect of VPA spans from maximal electroshock seizures through partial seizures to all types of generalized seizures; in the clinical setting, although VPA is effective in all types of epilepsies it appears to be more effective in generalized than in focal epilepsies (Loscher, 2002). In the case of IGEs, several mechanisms may be contributing to this drug’s efficacy, depending on the site of action and the molecular and cellular mechanisms that underlie absence, myoclonic, and generalized seizures (Loscher, 2002). These mechanisms include potentiation of c-aminobutyric acid (GABA) functions via increased GABA concentration, negative modulation of neuronal excitation via its effects on N-methyl-D-aspartate (NMDA) receptors, and probably the most important for the control of generalized seizures (especially absence) effects on hydroxybutyrate (GHB) (Loscher, 2002). Therefore, if we consider IGEs through the theories that postulate the corticoreticular mechanism of IGEs (and the abnormal oscillations within the network), it becomes obvious that the relatively wide distribution of these receptors in the mammalian brain predisposes VPA to being the archetypical drug for these IGEs because of its mechanisms of action (Gloor, 1968; Gloor et al., 1977). Although GABA receptors in the mammalian brain may be distributed somewhat more centrally then peripherally including thalami and basal ganglia (Young & Chu, 1990; Sieghart & Sperk, 2002), NMDA and GHB receptors appear to be distributed throughout the brain including thalami and cortex (especially prefrontal areas) (Monaghan & Cotman, 1985; Hechler et al., 1992). Therefore, the receptors via which VPA appears to exert its mechanism are distributed in all parts of the corticoreticular network, probably facilitating response to this AED. However, if the mechanisms of some of the seizures in some of the patients with IGEs are cortical, as postulated by some authorities (e.g., due to cortical micromalformations), then the pathophysiologic mechanisms that lead to these seizures may be mediated by different mechanisms, thus making VPA less efficacious (Niedermeyer et al., 1969b; Meencke & Janz, 1984; Dichter & Ayala, 1987; Niedermeyer, 1996). This could be the case with the patients who did not respond to VPA.
Our group analysis of the EEG/fMRI correlates of the GSWDs (Fig. 1 – left) showed fairly typical distribution of the positive and negative BOLD signal changes including early BOLD signal increases in thalami and basal ganglia and bilateral frontotemporal cortices (Salek-Haddadi et al., 2003; Aghakhani et al., 2004; Gotman et al., 2005; Moeller et al., 2008, 2009; Bai et al., 2010; Szaflarski et al., 2010a), and later BOLD signal decreases in the areas typical of the DMN (Gotman et al., 2005; Fox & Raichle, 2007; Morgan et al., 2008; Kay et al., 2012). However, the comparison between VPA-resistant and VPA-responsive TGE patients paints a very different picture (Fig. 1 – right). There are differences between patient groups with VPA-resistant patients clearly having more cortical involvement in GSWD generation, including bilateral insula in proximity to face/oral sensorimotor cortex, medial prefrontal, and frontal areas (anterior portion of the cingulate gyrus) when compared to VPA-responsive patients; these patients also exhibit different timing of the BOLD signal changes (Figs. 3 and 4). Furthermore, these differences are consistently present throughout the time course of BOLD signal response between −4 and +4 s centered on the HRF. There is only one very small area of increased BOLD signal in the VPA-responsive group when compared to the VPA-resistant group in the deep white matter of the frontal lobe at +2 s after the peak of the HRF, which may be suggestive of possibly greater involvement of DMN in patients with VPA-responsive IGEs. Could the reason for VPA-resistance in these patients be the fact that they have different pathophysiologic (focal cortical) mechanism of their IGE? These results certainly are in agreement with the possible reasons for VPA resistance discussed above; they are also discussed below in view of the previously proposed theories of the GSWD sources, and the available animal and human data.
Rodent studies in genetic models of generalized epilepsy provide evidence of Na+- and/or Ca++-channel mutations as the etiology of absence epilepsy (Tsakiridou et al., 1995; Crunelli & Leresche, 2002; Richards et al., 2003). In addition, recent rodent studies indicate that the thalamus and its connections are essential for the production of generalized GSWDs and their characteristic morphology (Futatsugi & Riviello, 1998; Blumenfeld & McCormick, 2000). However, systemic administration of ethosuximide in the genetic rat model of absence epilepsy (AED with well-recognized antiabsence properties; Glauser et al., 2010) reduced the firing rate of nucleus reticularis thalami by 90%, with the response much slower and of lower magnitude when it was administered directly into the thalamus (Richards et al., 2003). This suggests that the thalamus might not be the only source of GSWDs, and that the participation of the cortical structures in generation of the generalized GSWDs remains likely, which is in support of the “cortical” and “cortical focus” theories of IGE onset (Niedermeyer, 1996; Meeren et al., 2005).
One of the early animal EEG/fMRI studies showed BOLD signal changes in both cortical and thalamic structures in response to absence seizures (Tenney et al., 2004). Since then, several animal and human studies have shown various patterns of BOLD signal changes in response to GSWDs and/or absence seizures (Aghakhani et al., 2004; Gotman et al., 2005; Hamandi et al., 2006; Moeller et al., 2009, 2008; Bai et al., 2010; Szaflarski et al., 2010a). Therefore, it still remains to be determined whether it is the thalamus, the cortex, or an interaction between them that are responsible for seizure onset in IGEs. Although the thalamus, with its connections to all areas of the cortex and its baseline rhythmic firing of bursts of action potentials is an important, if not the most important, part of the network that sustains GSWDs, it is possible that the cortical sources contribute to the etiology of generalized epilepsies (Blumenfeld, 2002), with the last notion being consistent with the corticoreticular theory by Gloor (Gloor et al., 1977); Quesney et al., 1977; Avoli & Gloor, 1981; Avoli et al., 1981). Knowing that the entire network is necessary for GSWD production allows us to speculate that dysfunction or malfunction of different components of the network may contribute to different clinical presentation of the IGEs and, thus, to different responses to AEDs (Andermann & Berkovic, 2001). Our EEG/fMRI data certainly support the possibility that different portions of the network may participate in GSWD generation in patients who do or do not respond to VPA.
Possible limitations of this study need to be discussed. First, the definitions of “poor response” and “good response” to VPA are based on chart review and not on prospective data collection, and are subject to patient recall and physician record bias. In addition, these measures are far from perfect—a much better measure would be seizure freedom documented prospectively via ambulatory EEG and a longer span of seizure freedom, for example, 1 year. However, as discussed previously, this measure is impractical, as many charts do not contain such data (Szaflarski et al., 2008). Second, patient selection for study participation was based on fulfilling all inclusion criteria including at least one abnormal EEG with GSWDs. However, many patients with IGEs have very few EEG abnormalities and some of them may always have normal EEGs; normal EEGs are more frequently seen in patients with good response to AEDs, including VPA (Szaflarski et al., 2010b). Furthermore, the proportion of patients who had normal EEG during the EEG/fMRI procedure is higher than anticipated. Therefore, the possibility of inclusion/exclusion bias cannot be excluded, although it appears to be unlikely. Finally, this study was neither designed nor powered to answer the question as to whether the VPA-resistant patients responded better to AEDs typically used in patients with focal epilepsies. Although such a response was not seen in another larger study, a prospective data collection is needed to answer such question (Szaflarski et al., 2010b).
ACKNOWLEDGMENTS
This study was supported in part by K23 NS052468 to JPS and in part by funds from the Departments of Neurology at the University of Cincinnati, Cincinnati, OH. This study was presented in part at the Annual Meeting of the Organization of Human Brain Mapping in Beijing, China, 6/2012.
APPENDIX A
Diagnosis of IGE was based on criteria provided by a number of authors (Aliberti et al., 1994; Bourgeois et al., 1987; Fernando-Dongas et al., 2000; Gelisse et al., 2001; Genton & Gelisse, 2001; Lancman et al., 1994; Panayiotopoulos, 1989, 2005; Resor & Resor, 1990; Sadleir et al., 2009; Szaflarski, 2004; Szaflarski et al., 2010a,b):
Inclusion criteria:
History of two or more unprovoked seizures;
Clinical criteria of myoclonic jerks, typical absence seizures, and/or generalized tonic–clonic seizures;
Typical for IGE syndrome age of seizure onset;
For JME, circadian distribution of myoclonic jerks (on awakening) and characteristic precipitating factors such as sleep deprivation and/or alcohol consumption;
Normal general and neurologic examination;
Normal structural 1.5T or 3T MRI;
Characteristic, abnormal EEG with GSWDs and poly-SWDs with bifrontal predominance at a frequency of 3–6 Hz superimposed on a normal background;
Provision of a written informed consent by the subject (in case of minors—assent by the subject and written consent by the parent/guardian);
Age between 15 and 65.
Exclusion criteria:
Underlying degenerative or metabolic disorders or super-vening medical illness;
Abnormal general or neurologic examination;
Abnormal MRI of the brain;
Alcohol/drug abuse, medication noncompliance, lack of sleep hygiene, etc., leading to “VPA pseudo-resistance”;
Severe depression or other psychiatric disorders (e.g., psychosis) determined based on the previous medical history and/or the results of psychiatric consultation/follow-up;
Positive pregnancy test;
Any contraindication to an MRI scan at 4T;
Mental handicap (full-scale intelligence quotient [FSIQ] < 80 if tested in the Epilepsy Monitoring Unit) or history of special education and/or not being able to finish high school.
Definitions of VPA-responsive and VPA-resistant IGE are based on the available literature (Aliberti et al., 1994; Bourgeois et al., 1987; Fernando-Dongas et al., 2000; Gelisse et al., 2001; Genton and Gelisse, 2001; Lancman et al., 1994; Panayiotopoulos, 1989; Panayiotopoulos, 2005; Resor and Resor, 1990; Sadleir et al., 2009; Szaflarski, 2004; Szaflarski et al., 2010a,b). They are as follows:
Definition of VPA-response (good response):
Meeting inclusion criteria for IGE as described above;
Achievement of seizure freedom within 1 year of initiating therapy with valproate (usually seizure freedom will have been obtained in a shorter time frame);
Documentation of a minimum valproate serum level of 30 μg/ml while seizure free;
Sustained seizure freedom for at least 3 months.
Definition of VPA-resistance (poor response):
Meeting inclusion criteria for IGE as described above;
One or more seizures with a documented valproate serum level of >70 μg/ml, and without evidence of a significant proximate cause for the seizure such as head trauma with loss of consciousness or central nervous system infection.
Footnotes
DISCLOSURE
The authors declare no conflicts of interest. We also confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- Aghakhani Y, Bagshaw AP, Benar CG, Hawco C, Andermann F, Dubeau F, Gotman J. fMRI activation during spike and wave discharges in idiopathic generalized epilepsy. Brain. 2004;127:1127–1144. doi: 10.1093/brain/awh136. [DOI] [PubMed] [Google Scholar]
- Aliberti V, Grunewald R, Panayiotopoulos C, Chroni E. Focal electroencephalographic abnormalities in juvenile myoclonic epilepsy. Epilepsia. 1994;35:297–301. doi: 10.1111/j.1528-1157.1994.tb02433.x. [DOI] [PubMed] [Google Scholar]
- Allen PJ, Josephs O, Turner R. A method for removing imaging artifact from continuous EEG recorded during functional MRI. Neuroimage. 2000;12:230–239. doi: 10.1006/nimg.2000.0599. [DOI] [PubMed] [Google Scholar]
- Andermann F, Berkovic SF. Idiopathic generalized epilepsy with generalized and other seizures in adolescence. Epilepsia. 2001;42:317–320. doi: 10.1046/j.1528-1157.2001.36400.x. [DOI] [PubMed] [Google Scholar]
- Andersson J, Jenkinson M, Smith S. FMRIB technical report TR07JAI. 2007a Available at: www.fmrib.ox.ac.uk/analysis/techrep (Accessed July 11, 2012)
- Andersson J, Jenkinson M, Smith S. Non-linear registration, aka Spatial normalisation FMRIB technical report TR07JA2. 2007b Available at: www.fmrib.ox.ac.uk/analysis/techrep (Accessed July 11, 2012)
- Avoli M. A brief history on the oscillating roles of thalamus and cortex in absence seizures. Epilepsia. 2012;53:779–789. doi: 10.1111/j.1528-1167.2012.03421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, Gloor PV. The effects of transient functional depression of the thalamus on spindles and on bilateral synchronous epileptic discharges of feline generalized penicillin epilepsy. Epilepsia. 1981;22:443–452. doi: 10.1111/j.1528-1157.1981.tb06155.x. [DOI] [PubMed] [Google Scholar]
- Avoli M, Siatitsas I, Kostopoulos G, Gloor P. Effects of post-ictal depression on experimental spike and wave discharges. Electroencephalogr Clin Neurophysiol. 1981;52:372–374. doi: 10.1016/0013-4694(81)90066-3. [DOI] [PubMed] [Google Scholar]
- Bai X, Vestal M, Berman R, Negishi M, Spann M, Vega C, Desalvo M, Novotny EJ, Constable RT, Blumenfeld H. Dynamic time course of typical childhood absence seizures: EEG, behavior, and functional magnetic resonance imaging. J Neurosci. 2010;30:5884–5893. doi: 10.1523/JNEUROSCI.5101-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baykan B, Altindag EA, Bebek N, Ozturk AY, Aslantas B, Gurses C, Baral-Kulaksizoglu I, Gokyigit A. Myoclonic seizures subside in the fourth decade in juvenile myoclonic epilepsy. Neurology. 2008;70:2123–2129. doi: 10.1212/01.wnl.0000313148.34629.1d. [DOI] [PubMed] [Google Scholar]
- Berg AT, Shinnar S, Levy SR, Testa FM, Smith-Rapaport S, Beckerman B. Early development of intractable epilepsy in children: a prospective study. Neurology. 2001;56:1445–1452. doi: 10.1212/wnl.56.11.1445. [DOI] [PubMed] [Google Scholar]
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, Engel J, French J, Glauser TA, Mathern GW, Moshe SL, Nordli D, Plouin P, Scheffer IE. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H. The thalamus and seizures. Arch Neurol. 2002;59:135–137. doi: 10.1001/archneur.59.1.135. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, McCormick DA. Corticothalamic inputs control the pattern of activity generated in thalamocortical networks. J Neurosci. 2000;20:5153–5162. doi: 10.1523/JNEUROSCI.20-13-05153.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois B, Beaumanoir A, Blajev B, de la Cruz N, Despland P, Egli M, Geudelin B, Kaspar U, Ketz E, Kronauer C, Meyer C, Scollo-Lavizzari G, Tosi F, Vassella F, Zagury S. Monotherapy with valproate in primary generalized epilepsies. Epilepsia. 1987;28(Suppl. 2):S8–S11. doi: 10.1111/j.1528-1157.1987.tb05769.x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. The thalamic clock: emergent network properties. Neuroscience. 1991;41:351–364. doi: 10.1016/0306-4522(91)90332-i. [DOI] [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Commission Proposal for revised clinical and electroencephalographic classification of epileptic seizures. From the Commission on Classification and Terminology of the International league Against Epilepsy. Epilepsia. 1981;22:489–501. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- Commission Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1989;30:389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crunelli V, Leresche N. Childhood absence epilepsy: genes, channels, neurons and networks. Nat Rev Neurosci. 2002;3:371–382. doi: 10.1038/nrn811. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dichter MA, Ayala GF. Cellular mechanisms of epilepsy: a status report. Science. 1987;237:157–164. doi: 10.1126/science.3037700. [DOI] [PubMed] [Google Scholar]
- Difrancesco MW, Holland SK, Szaflarski JP. Simultaneous EEG/functional magnetic resonance imaging at 4 Tesla: correlates of brain activity to spontaneous alpha rhythm during relaxation. J Clin Neurophysiol. 2008;25:255–264. doi: 10.1097/WNP.0b013e3181879d56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duewell S, Wolff SD, Wen H, Balaban RS, Jezzard P. MR imaging contrast in human brain tissue: assessment and optimization at 4 T. Radiology. 1996;199:780–786. doi: 10.1148/radiology.199.3.8638005. [DOI] [PubMed] [Google Scholar]
- Espay AJ, Schmithorst VJ, Szaflarski JP. Chronic isolated hemifacial spasm as a manifestation of epilepsia partialis continua. Epilepsy Behav. 2008;12:332–336. doi: 10.1016/j.yebeh.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando-Dongas MC, Radtke RA, VanLandingham KE, Husain AM. Characteristics of valproic acid resistant juvenile myoclonic epilepsy. Seizure. 2000;9:385–388. doi: 10.1053/seiz.2000.0432. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Futatsugi Y, Riviello JJ., Jr Mechanisms of generalized absence epilepsy. Brain Dev. 1998;20:75–79. doi: 10.1016/s0387-7604(97)00107-1. [DOI] [PubMed] [Google Scholar]
- Gelisse P, Genton P, Thomas P, Rey M, Samuelian JC, Dravet C. Clinical factors of drug resistance in juvenile myoclonic epilepsy. J Neurol Neurosurg Psychiatry. 2001;70:240–243. doi: 10.1136/jnnp.70.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genton P, Gelisse P. Antimyoclonic effect of levetiracetam. Epileptic Disord. 2000;2:209–212. [PubMed] [Google Scholar]
- Glauser TA, Cnaan A, Shinnar S, Hirtz DG, Dlugos D, Masur D, Clark PO, Capparelli EV, Adamson PC, Childhood Absence Epilepsy Study Group Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy. N Engl J Med. 2010;362:790–799. doi: 10.1056/NEJMoa0902014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor P. Generalized cortico-reticular epilepsies. Some considerations on the pathophysiology of generalized bilaterally synchronous spike and wave discharge. Epilepsia. 1968;9:249–263. doi: 10.1111/j.1528-1157.1968.tb04624.x. [DOI] [PubMed] [Google Scholar]
- Gloor P, Quesney LF, Zumstein H. Pathophysiology of generalized penicillin epilepsy in the cat: the role of cortical and subcortical structures. II. Topical application of penicillin to the cerebral cortex and to subcortical structures. Electroencephalogr Clin Neurophysiol. 1977;43:79–94. doi: 10.1016/0013-4694(77)90198-5. [DOI] [PubMed] [Google Scholar]
- Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F. Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc Natl Acad Sci U S A. 2005;102:15236–15240. doi: 10.1073/pnas.0504935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamandi K, Salek-Haddadi A, Laufs H, Liston A, Friston K, Fish DR, Duncan JS, Lemieux L. EEG-fMRI of idiopathic and secondarily generalized epilepsies. Neuroimage. 2006;31:1700–1710. doi: 10.1016/j.neuroimage.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Hechler V, Gobaille S, Maitre M. Selective distribution pattern of gamma-hydroxybutyrate receptors in the rat forebrain and midbrain as revealed by quantitative autoradiography. Brain Res. 1992;572:345–348. doi: 10.1016/0006-8993(92)90498-x. [DOI] [PubMed] [Google Scholar]
- Iqbal N, Caswell HL, Hare DJ, Pilkington O, Mercer S, Duncan S. Neuropsychological profiles of patients with juvenile myoclonic epilepsy and their siblings: a preliminary controlled experimental video-EEG case series. Epilepsy Behav. 2009;14:516–521. doi: 10.1016/j.yebeh.2008.12.025. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kay BP, Meng X, Difrancesco MW, Holland SK, Szaflarski JP. Moderating effects of music on resting state networks. Brain Res. 2012;1447:53–64. doi: 10.1016/j.brainres.2012.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerlund TD, Sharbrough FW, Busacker NE. Spatial filtering of multichannel electroencephalographic recordings through principal component analysis by singular value decomposition. J Clin Neurophysiol. 1997;14:73–82. doi: 10.1097/00004691-199701000-00007. [DOI] [PubMed] [Google Scholar]
- Lancman M, Asconape J, Penry J. Clinical and EEG asymmetries in juvenile myoclonic epilepsy. Epilepsia. 1994;35:302–306. doi: 10.1111/j.1528-1157.1994.tb02434.x. [DOI] [PubMed] [Google Scholar]
- Loscher W. Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs. 2002;16:669–694. doi: 10.2165/00023210-200216100-00003. [DOI] [PubMed] [Google Scholar]
- Marson AG, Al-Kharusi AM, Alwaidh M, Appleton R, Baker GA, Chadwick DW, Cramp C, Cockerell OC, Cooper PN, Doughty J, Eaton B, Gamble C, Goulding PJ, Howell SJ, Hughes A, Jackson M, Jacoby A, Kellett M, Lawson GR, Leach JP, Nicolaides P, Roberts R, Shackley P, Shen J, Smith DF, Smith PE, Smith CT, Vanoli A, Williamson PR, SANAD Study group The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalised and unclassifiable epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369:1016–1026. doi: 10.1016/S0140-6736(07)60461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meencke HJ, Janz D. Neuropathological findings in primary generalized epilepsy: a study of eight cases. Epilepsia. 1984;25:8–21. doi: 10.1111/j.1528-1157.1984.tb04149.x. [DOI] [PubMed] [Google Scholar]
- Meeren H, van Luijtelaar G, Lopes da Silva F, Coenen A. Evolving concepts on the pathophysiology of absence seizures: the cortical focus theory. Arch Neurol. 2005;62:371–376. doi: 10.1001/archneur.62.3.371. [DOI] [PubMed] [Google Scholar]
- Moeller F, Siebner HR, Wolff S, Muhle H, Granert O, Jansen O, Stephani U, Siniatchkin M. Simultaneous EEG-fMRI in drug-naive children with newly diagnosed absence epilepsy. Epilepsia. 2008;49:1510–1519. doi: 10.1111/j.1528-1167.2008.01626.x. [DOI] [PubMed] [Google Scholar]
- Moeller F, Siebner HR, Ahlgrimm N, Wolff S, Muhle H, Granert O, Boor R, Jansen O, Gotman J, Stephani U, Siniatchkin M. fMRI activation during spike and wave discharges evoked by photic stimulation. Neuroimage. 2009;48:682–695. doi: 10.1016/j.neuroimage.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Monaghan DT, Cotman CW. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985;5:2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan VL, Gore JC, Szaflarski JP. Temporal clustering analysis: what does it tell us about the resting state of the brain? Med Sci Monit. 2008;14:CR345–CR352. [PMC free article] [PubMed] [Google Scholar]
- Niedermeyer E. Primary (idiopathic) generalized epilepsy and underlying mechanisms. Clin Electroencephalogr. 1996;27:1–21. doi: 10.1177/155005949602700103. [DOI] [PubMed] [Google Scholar]
- Niedermeyer E, Laws ER, Jr, Walker AE. Depth EEG findings in epileptics with generalized spike-wave complexes. Arch Neurol. 1969a;21:51–58. doi: 10.1001/archneur.1969.00480130065007. [DOI] [PubMed] [Google Scholar]
- Niedermeyer E, Laws ER, Jr, Walker AE, Blumer D, Ray CD. The contribution of scalp and depth EEG findings to the surgical treatment of temporal lobe epilepsy. Electroencephalogr Clin Neurophysiol. 1969b;26:110. [PubMed] [Google Scholar]
- Panayiotopoulos C. Juvenile myoclonic epilepsy: An autosomal recessive disease. Ann Neurol. 1989;25:440–443. doi: 10.1002/ana.410250504. [DOI] [PubMed] [Google Scholar]
- Panayiotopoulos CP. Idiopathic generalized epilepsies: A review and modern approach. Epilepsia. 2005;46(Suppl 9):1–6. doi: 10.1111/j.1528-1167.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- Penfield W, Jasper H. Epilepsy and the functional anatomy of the human brain. Little Brown; Boston, MA: 1954. [Google Scholar]
- Quesney LF, Gloor P, Kratzenberg E, Zumstein H. Pathophysiology of generalized penicillin epilepsy in the cat: the role of cortical and subcortical structures. I. Systemic application of penicillin. Electroencephalogr Clin Neurophysiol. 1977;42:640–655. doi: 10.1016/0013-4694(77)90281-4. [DOI] [PubMed] [Google Scholar]
- Resor S, Resor L. The neuropharmacology of juvenile myoclonic epilepsy. Clin Neuropharmacol. 1990;13:465–491. doi: 10.1097/00002826-199012000-00001. [DOI] [PubMed] [Google Scholar]
- Richards DA, Manning JP, Barnes D, Rombola L, Bowery NG, Caccia S, Leresche N, Crunelli V. Targeting thalamic nuclei is not sufficient for the full anti-absence action of ethosuximide in a rat model of absence epilepsy. Epilepsy Res. 2003;54:97–107. doi: 10.1016/s0920-1211(03)00060-3. [DOI] [PubMed] [Google Scholar]
- Sadleir LG, Scheffer IE, Smith S, Carstensen B, Farrell K, Connolly MB. EEG features of absence seizures in idiopathic generalized epilepsy: Impact of syndrome, age, and state. Epilepsia. 2009;50:1572–1578. doi: 10.1111/j.1528-1167.2008.02001.x. [DOI] [PubMed] [Google Scholar]
- Salek-Haddadi A, Lemieux L, Merschhemke M, Friston KJ, Duncan JS, Fish DR. Functional magnetic resonance imaging of human absence seizures. Ann Neurol. 2003;53:663–667. doi: 10.1002/ana.10586. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Dardzinski BJ, Holland SK. Simultaneous correction of ghost and geometric distortion artifacts in EPI using a multiecho reference scan. IEEE Trans Med Imaging. 2001;20:535–539. doi: 10.1109/42.929619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneviratne U, Cook M, D’Souza W. The electroencephalogram of idiopathic generalized epilepsy. Epilepsia. 2012;53:234–248. doi: 10.1111/j.1528-1167.2011.03344.x. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP. Effects of zonisamide on the electroencephalogram of a patient with juvenile myoclonic epilepsy. Epilepsy Behav. 2004;5:1024–1026. doi: 10.1016/j.yebeh.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Rackley AY, Lindsell CJ, Szaflarski M, Yates SL. Seizure control in patients with epilepsy: the physician vs. medication factors. BMC Health Serv Res. 2008;8:264. doi: 10.1186/1472-6963-8-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, DiFrancesco M, Hirschauer T, Banks C, Privitera MD, Gotman J, Holland SK. Cortical and subcortical contributions to absence seizure onset examined with EEG/fMRI. Epilepsy Behav. 2010a;18:404–413. doi: 10.1016/j.yebeh.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Lindsell CJ, Zakaria T, Banks C, Privitera MD. Seizure control in patients with idiopathic generalized epilepsies: EEG determinants of medication response. Epilepsy Behav. 2010b;17:525–530. doi: 10.1016/j.yebeh.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenney JR, Duong TQ, King JA, Ferris CF. fMRI of brain activation in a genetic rat model of absence seizures. Epilepsia. 2004;45:576–582. doi: 10.1111/j.0013-9580.2004.39303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiridou E, Bertollini L, de Curtis M, Avanzini G, Pape HC. Selective increase in T-type calcium conductance of reticular thalamic neurons in a rat model of absence epilepsy. J Neurosci. 1995;15:3110–3117. doi: 10.1523/JNEUROSCI.15-04-03110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyvaert L, Chassagnon S, Sadikot A, LeVan P, Dubeau F, Gotman J. Thalamic nuclei activity in idiopathic generalized epilepsy: an EEG-fMRI study. Neurology. 2009;73:2018–2022. doi: 10.1212/WNL.0b013e3181c55d02. [DOI] [PubMed] [Google Scholar]
- Ugurbil K, Hu X, Chen W, Zhu XH, Kim SG, Georgopoulos A. Functional mapping in the human brain using high magnetic fields. Philos Trans R Soc Lond B Biol Sci. 1999;354:1195–1213. doi: 10.1098/rstb.1999.0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf P, Inoue Y. Therapeutic response of absence seizures in patients of an epilepsy clinic for adolescents and adults. J Neurol. 1984;231:225–229. doi: 10.1007/BF00313944. [DOI] [PubMed] [Google Scholar]
- Young A, Chu D. Distribution of GABAA and GABAB receptors in mammalian brain: potential targets for drug development. Drug Dev Res. 1990;21:161–167. [Google Scholar]