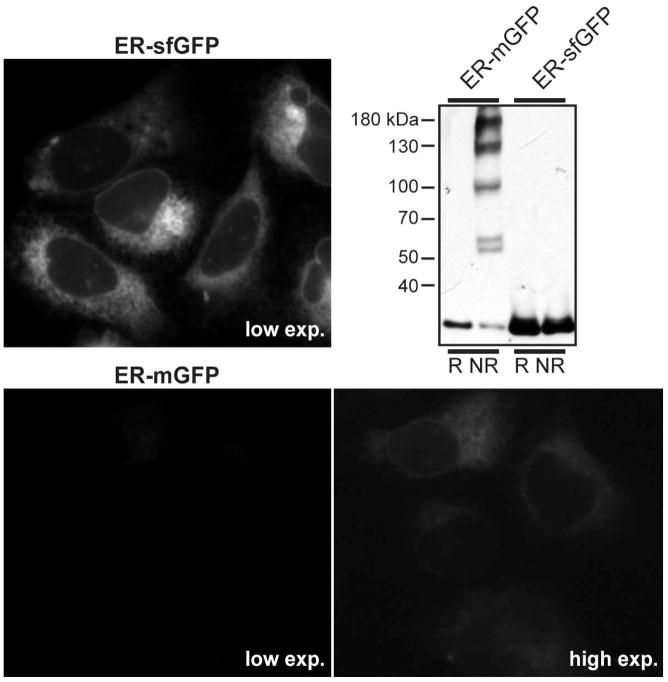
Figure 4. GFP Variants in the ER.
ER-sfGFP and ER-mGFP constructs were transiently transfected in U2-OS cells. While both constructs clearly localize to an ER pattern of networked tubules and the nuclear envelope, large differences in fluorescence intensity are apparent. Cells were imaged on a Zeiss Axiovert 200 microscope with 63X NA 1.4 objective with the same camera settings at two different exposures. At the low exposure, mGFP is not visible, while sfGFP is intensely fluorescent in the majority of cells. mGFP becomes visible only at a much longer exposure. In an immunoblot of Cos-7 cell lysates, the propensity of mGFP to oligomerize in the ER is apparent by the laddering observed in the nonreducing lane. In contrast, no laddering is apparent for sfGFP. Note how much of the mGFP remains monomeric (the lowest band) in the nonreducing gel. Only this pool of protein can still form a β-barrel and potentially fluoresce.