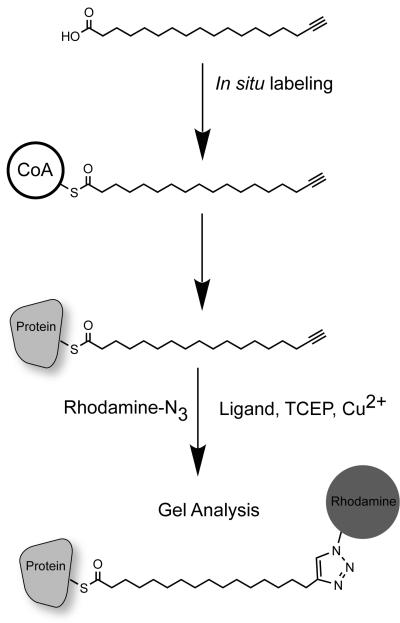
Figure 1.
Schematic of 17-ODYA metabolic labeling and detection of palmitoylated proteins. The commercially available alkynyl fatty acid 17-ODYA is added to cells in culture. Over the course of several hours, the probe is conjugated to CoA, and can serve as a substrate for protein palmitoyl transferases. The cells are then lysed and mixed with click chemistry reagents. The TCEP reduces the Cu(II) to Cu(I), which is bound to the TBTA ligand. Addition of azide-linked reporters, such as rhodamine-azide or biotin-azide, facilitate click chemistry conjugation and triazole formation, covalently linking the reporter to 17-ODYA thioesters on proteins. The labeled palmitoylated proteins are then separated by SDS-PAGE separation or affinity purified for mass spectrometry-based proteomics.