Abstract
Study Objectives:
To describe the time structure of leg movements (LM) in obstructive sleep apnea (OSA) syndrome, in order to advance understanding of their clinical significance.
Location:
Sleep Research Centre, Oasi Institute (IRCCS), Troina, Italy.
Setting:
Sleep laboratory.
Patients:
Eighty-four patients (16 females, 68 males, mean age 55.1 y, range 29-74 y).
Methods:
Respiratory-related leg movements (RRLM) and those unrelated to respiratory events (NRLM) were examined within diagnostic polysomnograms alone and together for their distributions within the sleep period and for their periodicity.
Measurements and Results:
Patients with OSA and RRLM exhibited more periodic leg movements in sleep (PLMS), particularly in NREM sleep. A gradual decrease in number of NRLM across the sleep period was observed in patients with RRLM. This pattern was less clear for RRLM. Frequency histograms of intermovement intervals of all LMs in patients with RRLM showed a prominent first peak at 4 sec, and a second peak at approximately 24 sec coincident with that of PLMS occurring in the absence of OSA. A third peak of lowest amplitude was the broadest with a maximum at approximately 42 sec. In patients lacking RRLM, NRLM were evident with a single peak at 2-4 sec. A stepwise linear regression analysis showed that, after controlling for a diagnosis of restless legs syndrome and apnea-hypopnea index, PLMS remained significantly associated with RRLM.
Conclusion:
The time structure of leg movements occurring in conjunction with respiratory events exhibit features of periodic leg movements in sleep occurring alone, only with a different and longer period. This brings into question the validity, both biologic and clinical, of scoring conventions with their a priori exclusion from consideration as periodic leg movements in sleep.
Citation:
Manconi M; Zavalko I; Bassetti CL; Colamartino E; Pons M; Ferri R. Respiratory-related leg movements and their relationship with periodic leg movements during sleep. SLEEP 2014;37(3):497-504.
Keywords: Periodicity index, periodic leg movements, respiratory-related leg movements, sleep apnea, time distribution
INTRODUCTION
Periodic leg movements in sleep (PLMS) are found in 24-48% of patients with obstructive sleep apnea (OSA).1,2 The current criteria for the scoring of a leg movement (LM) as a PLMS excludes those associated with respiratory events (i.e., respiratory-related leg movement; RRLM).3,4 The rationale underlying this convention seems to be that RRLM are provoked by respiratory-related arousals, and because of the periodic nature of obstructive respiratory events, they “mimic” PLMS. Thus, they are considered unique from PLMS that occur as part of the phenotypic spectrum of restless legs syndrome (RLS; i.e., “genuine” PLMS). This line of thinking has treatment implications, because the persistence of LMs with continuous positive air pressure (CPAP) treatment has been taken by some to be an indication of insufficient treatment pressure.5 During treatment of sleep related breathing disorders, PLMS may decrease or disappear, persist, or even increase.6,7 The variable response of PLMS to CPAP and the suspected refractoriness of RRLM to dopamine-agonists8 has been interpreted by some to mean that RRLM and PLMS have different pathogenetic mechanisms.
The coexistence of sleep disordered breathing and LM has not been comprehensively studied,1,2,9,10 but it has been suggested that sympathetic activations are significantly greater when the termination of apneas is associated with leg movements compared to those without leg movements.11 Moreover, it has been suggested that treatment interventions for sleep disordered breathing may influence the time structure of LM,12,13 but the case of a real comorbidity between OSA and RLS has not been posited as frequently; i.e., where “genuine” PLMS might synchronize with the respiratory events.
Thus, it can be said that the occurrence of RRLM might be influenced by at least three main factors: (1) the presence of PLMS not related to respiratory events; (2) the presence of RLS; and (3) the severity of the respiratory disturbance. The current investigation used an approach to the study of the time structure of LM during sleep in OSA that is able to characterize, in detail, the strength of periodicity to this motor phenomenon, together with its time distribution,14,15 in order to add new knowledge to the understanding of the biological origins and clinical relevance of LM observed during sleep in OSA.
METHODS
Patient Selection
A retrospective chart review of all patients visiting the sleep laboratory of the Sleep and Epilepsy Center of the Neurocenter of Southern Switzerland, from January 2010 to July 2011, was conducted. We selected subjects who had undergone full-night polysomnographic recording and who had an apnea-hypopnea index (AHI) > 20, an oxygen desaturation index (ODI) > 10, and more than half of all apneas classified as obstructive, and who were between 25 to 75 y of age. We excluded subjects with any known comorbid condition (such as narcolepsy, parasomnia, neurological disorders, or renal or heart failure) or on any medication (e.g., dopamine agonists, benzodiazepines, antidepressants), that might influence LM or apnea. Untreated RLS were included and RLS status was controlled for in subsequent analyses. Recordings with artifacts on the tibialis anterior or flow channels for more than 20% of the total sleep time were also excluded. The study protocol was approved by the local Ethics Committee.
Polysomnographic Recordings
All polysomnographic recordings included six electroencephalogram (EEG) channels (F3/A2, F4/A1, C3/A2, C4/ A1, O1/A2, O2/A1), submental electromyogram, electrooculogram, electrocardiogram, cardiorespiratory channels (nasal flow, breathing effort, pulsoximetry), and electromyogram of the right and left tibialis anterior muscles (bipolar derivations with two electrodes). All recordings were previously scored for clinical purposes by experienced medical doctors, following the standard criteria of the American Academy of Sleep Medicine (AASM).4 All recordings were transformed in European Data Format and LM were reanalyzed with the sleep analysis software Hypnolab 1.2 (SWS Soft, Italy). All LM were detected automatically and subsequently visually reviewed and classified by one investigator (IZ). In particular, RRLM were identified and excluded from the analysis of the standard LM activity parameters during sleep, following the WASM/IRLSSG criteria.3 The remaining LM were referred to as nonrespiratory leg movement (NRLM). Figure 1 shows examples of this classification process; in particular, RRLM are LM that overlap, precede, or are followed by less than 0.5 sec at the end of an apnea or hypopnea event.
Figure 1.
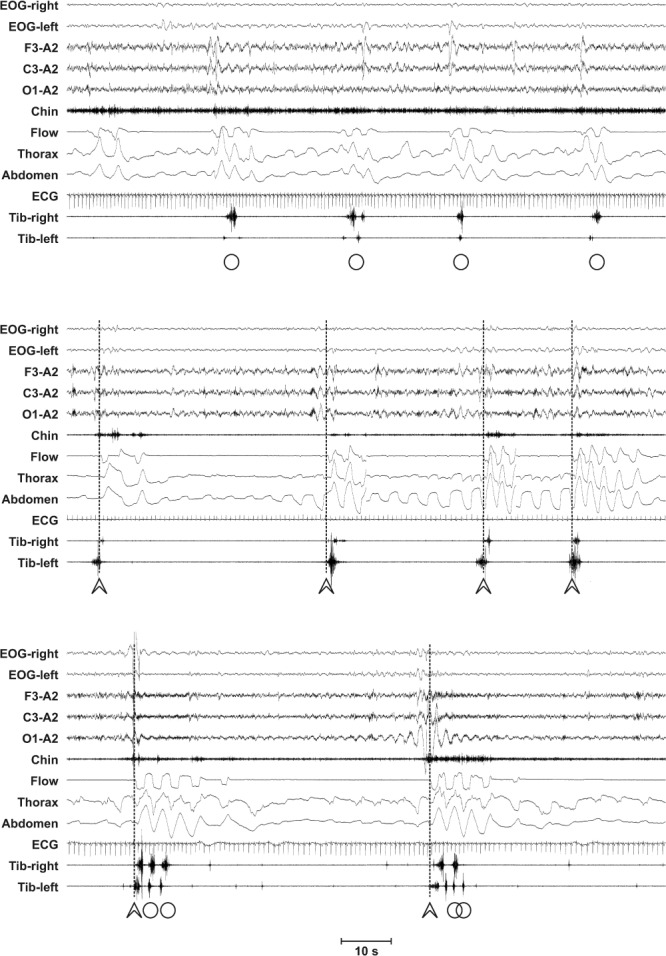
Examples of the leg movement (LM) detection process in our patients with obstructive sleep apnea. Arrowheads indicate LM associated with the resumption of breathing after an apnea-hypopnea event (vertical dotted lines), whereas circles indicate LM not associated to an apnea-hypopnea event Note the possible coexistence of both events in the period between two consecutive apnea-hypopnea events. EOG, electrooculogram.
LM parameters were computed separately for NRLM and RRLM: total LM index (total number of LM per hour of sleep) and PLMS index (total number of LM included in a series of four events or more, separated by more than five events and less than 90 sec, per hour of sleep). The analysis included the PLMS index of the entire night, and separately of rapid eye movement (REM) and nonrapid eye movement (NREM) sleep, as well as the total number of LM and number of PLMS sequences. Finally, the periodicity index (PI) was computed as the number of intervals belonging to sequences of at least three inter-LM intervals 10 < i ≤ 90 sec/total number of inter-LM intervals. This index can vary between 0 (absence of periodicity) to 1 (all intervals with length 10 < i ≤ 90 sec).14
Statistical Analysis
Group comparisons were carried out with the nonparametric Mann-Whitney U test for independent data sets. We also calculated effect sizes using the Cohen d value. Cohen d is defined as the difference between two means divided by their pooled standard deviation. According to this value, 0.2 is indicative of a small effect, 0.5 a medium effect, and 0.8 a large effect size. To control for the multiplicity of statistical tests and taking into consideration the nonindependence of multiple outcomes from the same domain, the Bonferroni-adjusted alpha level was set to 0.01 and 0.008, accounting for five or six independent dimensions, respectively, as specified for the different comparisons (Tables 1 through 3). The chi-square test was used for comparison of frequencies. Correlations were analyzed by means of the nonparametric Spearman rho coefficient. The complex distribution of inter-LM intervals was modeled with distribution mixture analysis. Finally, a stepwise linear regression was performed to understand the association of RRLM to other variables.
Table 1.
Comparison between clinical features, respiratory parameters, sleep architecture in patients with obstructive sleep apnea with (OSA LM+) or without (OSA LM-) leg movement > 15/h
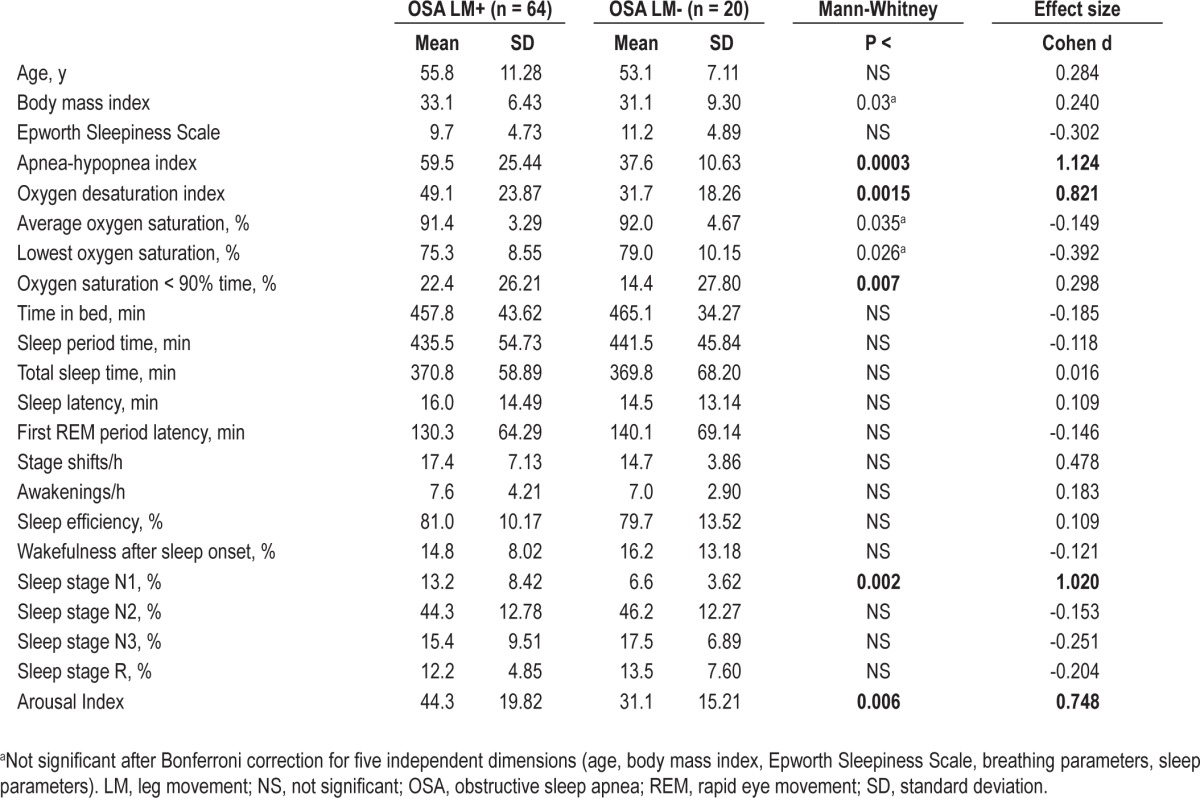
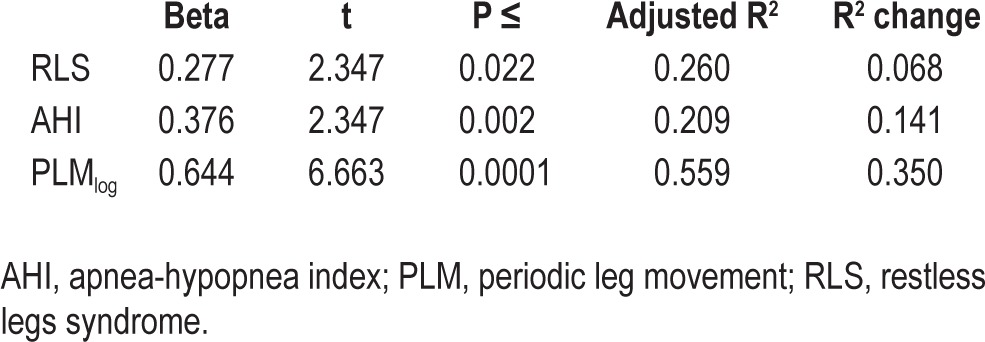
Table 3.
Stepwise linear regression with respiratory-related leg movement index as a dependent variable and periodic leg movements in sleep index, restless legs syndrome status, and apnea-hypopnea index as independent factors.
RESULTS
Among 682 patients who had undergone full polysomnographic recording in the relevant 19- mo period, 163 subjects met general inclusion criteria. We excluded 69 patients with medical condition and/or drug therapy known or suspected to influence LM or respiratory events, and 10 recordings with significant artifacts of the tibialis anterior or flow channels. The final study population included 84 patients (16 females, 68 males, mean age 55.1 y, 10.46 standard deviation, range, 29-74 y). Information on RLS status was available for 77 patients and 11 of them (14%) them fulfilled RLS diagnostic criteria.16
Characterization of LM Activity in the Entire Patient Group
We first selected a subgroup of patients with frequent LM, based on an arbitrary cutoff of > 15/h. Sixty-four patients fulfilled this criterion (55 males and 9 females) and 20 did not (13 males and 7 females). The male preponderance in the first group was statistically significant (chi-square 4.33, P = 0.037), and age was not (Table 1). Comparison of clinical features, respiratory parameters, and sleep architecture in patients with OSA with or without LM > 15/h is reported in Table 1; the subjects with total LM index > 15/h tended to have a higher body mass index and show more severe sleep apnea, in particular regarding the AHI and ODI. In addition, patients with increased LM also showed increased percentage of sleep stage N1 and a higher number of arousals.
Characterization of Patients With or Without RRLM
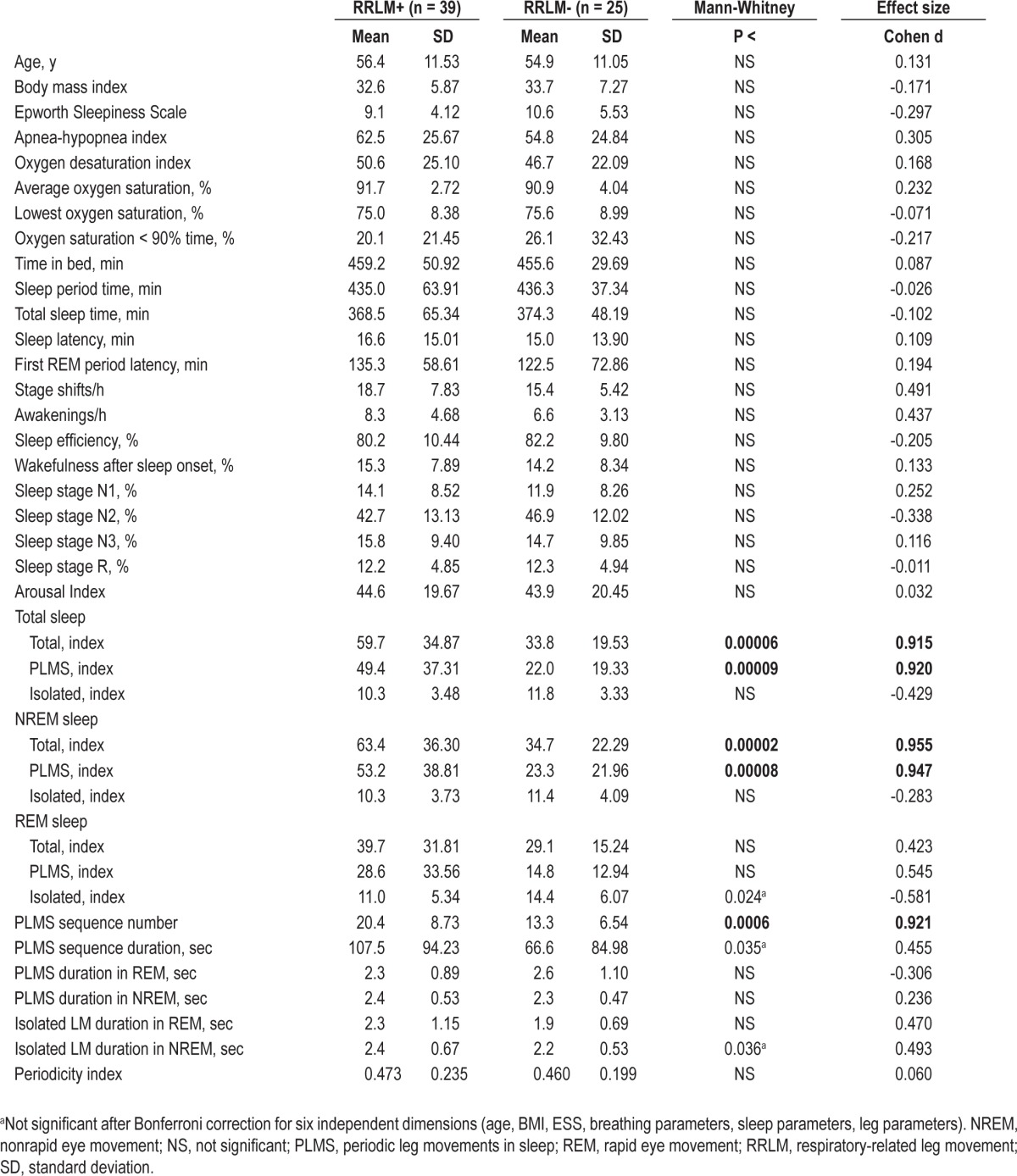
Of the 64 patients exhibiting frequent LMs (LM index > 15), further stratification was based on the presence or absence of RRLM > 5/h. Thirty-nine patients had > 5 RRLM/h (RRLM+; 33 males and 6 females), and 25 did not (22 males and 3 females). The sex distribution was not significantly different (chi-square 0.704, P = 0.14). Table 2 shows the comparison of clinical features, respiratory parameters, sleep architecture, and leg motor activity during sleep between patients with OSA with or without RRLM. Only leg movement parameters were found to differ between the groups, being significantly higher in RRLM+ subjects. The effect was strong for the total LMs and PLMS index, in particular during NREM sleep, and for the number of PLMS sequences.
Table 2.
Comparison between clinical features, respiratory parameters, sleep architecture and leg motor activity during sleep in patients with obstructive sleep apnea with or without respiratory-related leg movement
The intermovement interval distribution histograms of the different LM activities recorded in patients with OSA with or without RRLM are shown in Figure 2. In the top panel, the data of the RRLM+ patients are reported. In this panel, NRLM show a prominent first peak at 4 sec, followed by another at approximately 24 sec and extending up to approximately 60-70 sec. In the same group, RRLM show a unique, broader peak of lower magnitude, extending from approximately 20-70 sec and with a maximum at about 42 sec. The distribution in patients without RRLM is shown in the bottom panel of Figure 2. In these patients only NRLMs are evident, with a single peak at 2-4 sec.
Figure 2.
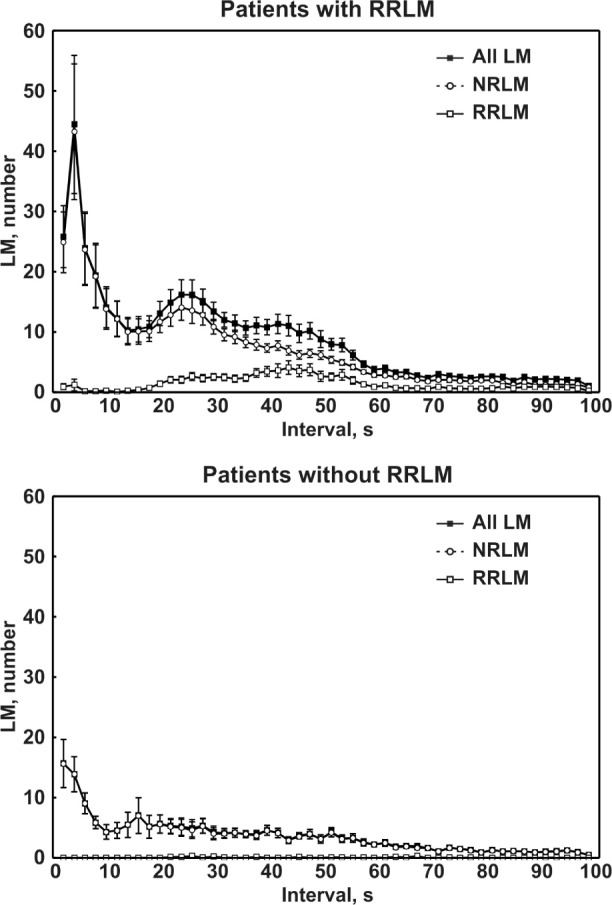
Intermovement interval distribution histograms of the different leg movement (LM) activities recorded in patients with obstructive sleep apnea with or without respiratory-related leg movement (RRLM). NRLM, nonrespiratory-related leg movement.
Subsequently, for the total number of LM, we attempted to model the observed inter-movements intervals in RRLM+ patients by means of a distribution mixture analysis using three separate lognormal distributions; this analysis is shown in the top panel of Figure 3. The bottom panel shows the residuals (difference between observed and expected values) that are very small across the entire intermovement interval range.
Figure 3.
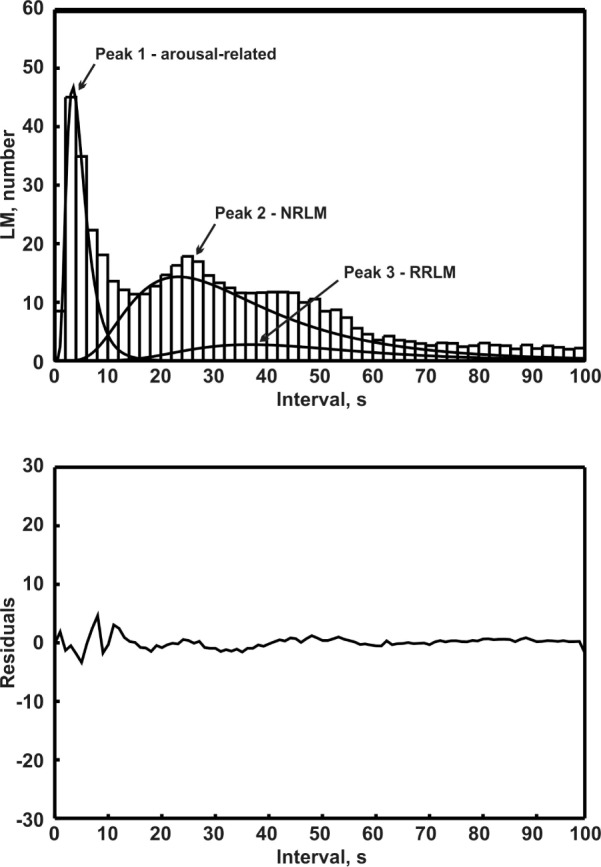
Distribution mixture analysis (lines) performed on the total intermovement interval distribution histogram (vertical bars) of LM activity recorded in the group of patients with OSA and respiratory-related leg movement (RRLM) (n = 39). NRLM, nonrespiratory-related leg movemens.
Figure 4 depicts the time course of the number of LM per hour of night for RRLM and NRLM recorded in RRLM+ patients (top panel) and in subjects without RRLM. Patients with RRLM+ show a gradual decrease in the number of NRLM, from the first to the last hours of sleep; this pattern was less clear but still detectable for RRLM in this group. No clear decremental pattern was evident for all LM types in the group without RRLM.
Figure 4.
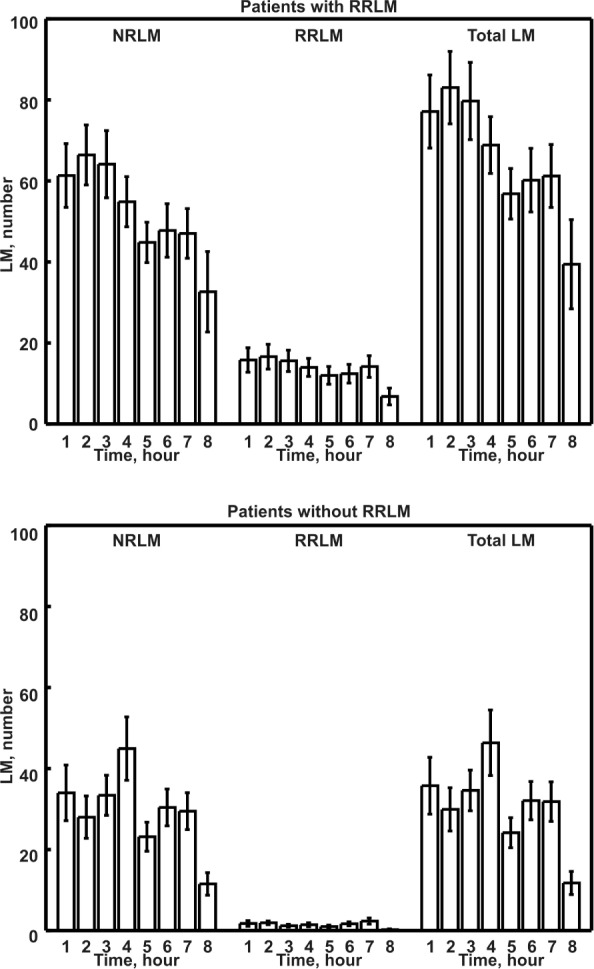
Number of leg movements (LM) per hour of night of the different LM activities recorded in patients with obstructive sleep apnea with or without respiratory-related leg movement (RRLM). NRLM, nonrespiratory-related leg movement.
The top panel of Figure 5 shows the intermovement interval distribution histograms of the different LM activities recorded in the subgroup of patients with OSA and RRLM with a diagnosis of RLS. These histograms are similar to those of the entire group of patients, with the expected peak at 18-20 sec, typically observed in patients with RLS without OSA,14,17–20 being greatly reduced. The bottom panel of Figure 5 shows the inter-movement interval distribution histograms of the different LM activities recorded in the subgroup of patients with OSA and RRLM but without RLS.
Figure 5.
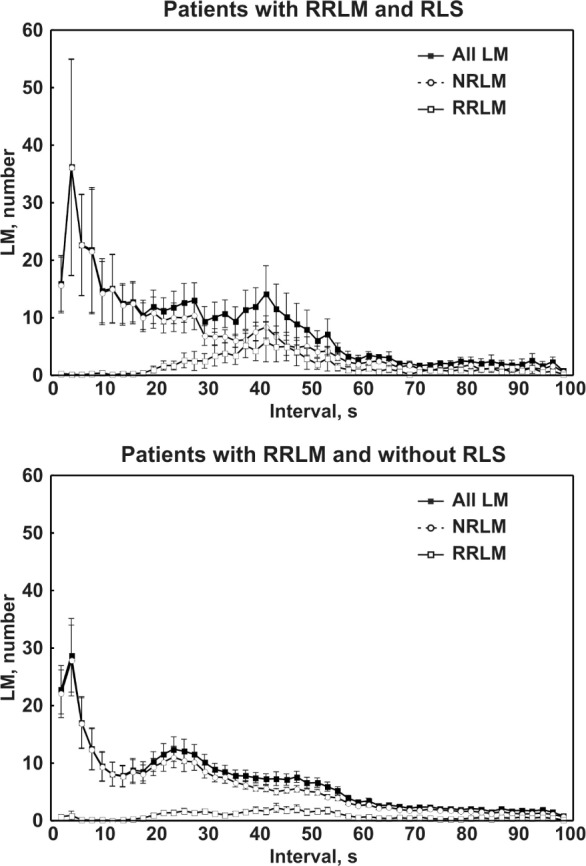
Top panel: Intermovement interval distribution histograms of the different leg movement (LM) activities recorded in the subgroup of patients with obstructive sleep apnea (OSA) with respiratory-related leg movement (RRLM) and restless legs syndrome (RLS). Bottom panel: Intermovement interval distribution histograms of the different LM activities recorded in the subgroup of patients with OSA with RRLM but without RLS. NRLM, nonrespiratory-related leg movement.
Given these findings and previous knowledge, it seemed plausible that the risk for the presence of RRLM might be influenced by three factors: PLMS frequency, AHI severity, and RLS status, with PLMS being associated both with AHI and RLS. In order to verify if PLMS were associated with RRLM independent of their relationship with AHI and presence of RLS, we computed a stepwise linear regression with RRLM index as the dependent variable and entering PLMS index only after controlling for its association with AHI and RLS. Because of concerns about the non-normal distribution of the RRLM index and PLMS index (Kolgomorov-Smirnov Z test of 1.868 and 1.350, P = 0.002 and 0.052, respectively) we log10-transformed both variables (Kolmogorov-Smirnov Z test: 0.930 and 0.610, P = 0.353 and 0.850, respectively). In the stepwise regression model, both AHI and RLS status were related to RRLM index (Table 3). Even when controlling for AHI and RLS status, PLMS were highly significantly associated with RRLM (incremental R2 change: 0.35, Table 3).
DISCUSSION
Our results show that individuals with RRLM consistently have NRLM with features typical for “genuine” PLMS. These features, which include a clear peak at approximately 20-24 sec in the intermovement interval histogram along with a gradually decreasing frequency during the night,15 have been consistently observed in RLS,14,17,21 PLMD,22 and, with different degrees, in other conditions such as narcolepsy23 or REM sleep behavior disorder.24 In the current study, patients with OSA in whom these “genuine” PLMS are not observed, RRLM also were not observed.
With the analysis of the intermovement interval distribution of RRLM, we have found that they show a peak partially overlapping with that of PLMS, even if with a somewhat longer average interval (42 versus 24 sec). This is in agreement with and explains the results of previous studies that reported similar information on both interapnea and intermovement intervals.12,13 Carelli et al.12 reported that intermovement intervals of PLMS were shorter than those of RRLM, both at baseline and under CPAP treatment. Briellmann et al.13 showed that LM with longer intermovement intervals disappeared under CPAP, whereas LM with shorter intermovement intervals persisted or even increased during CPAP therapy; this supports the hypothesis that a portion of “genuine” PLMS, which would have a typical period of approximately 20-24 sec in the absence of apnea events, might be captured and their period might be forced to follow the characteristic longer apnea time structure.25 The subsequent elimination of apnea might be followed by a persistence of PLMS, which would then return to their typical shorter periodicity. Finally, the time distribution of RRLM during the night also was similar to that of NRLM, with a less clear decreasing trend.
From the analysis of differences between several clinical features, sleep architecture, respiratory measures, and NRLM parameters found in patients with or without RRLM, we found that only LM parameters were clearly different and that almost all PLMS indicators were higher in patients with RRLM. This is in line with the idea that RRLM emerge in subjects with a genetically determined diathesis or susceptibility to express PLMS.26,27 In further support of this interpretation, we found that the correlation between RRLM and AHI was significant. An indirect confirmation might also come from a report on rates of RLS that are higher in OSA and revert to normal frequencies with treatment.28 We also suspect that RLS status might influence the occurrence of RRLM. For this reason, we performed a stepwise linear regression with RRLM index as the dependent variable, and PLMS index was entered only after controlling for the association with RLS status and AHI. Even in this instance, PLMS index associated with a high significance value to the presence of RRLM.
We should briefly mention that also in our analysis, in agreement with previous reports,2 we have found that the presence of LM during sleep in patients with OSA is associated with higher AHIs. Additionally, we also found a significant increase in some indexes of disrupted sleep (sleep stage N1 percentage and arousal index); however, subjective sleepiness was found to be similar in the two groups. These considerations might not be trivial because the finding of an important amount of LM activity during sleep occurs frequently. In agreement with previous reports,1,2 we have found that 76% of the total group of patients with OSA had a total LM index > 15, 46% had RRLM > 5/h, and 59.5% showed a combination of LM amount and PI not different from those expected for patients with RLS.22 Our results, therefore, might indicate that clinical differences exist between patients with or without RRLM; however, because this was not the focus of our study, further speculation is not warranted.
The major limitation of this study is the absence of a control condition under CPAP treatment. Such an analysis would be an important extension of this study. Even if overt apnea episodes are significantly decreased, it would also be critical to carefully assess for the persistence of more subtle respiratory events5 and for arousal-related activities.29 The duration of the CPAP treatment would be an additional key feature to control for, because it has been shown that sleep pattern reorganization after the start of this treatment occurs and is complex.29
It is also important to emphasize that the data presented here refer to a selected group of patients with moderate to severe OSA and LM > 15/h; it cannot be excluded that other patients might present different LM features. Interpretation of our data should also carefully take into consideration an additional important possible bias, independent from our methodological procedure and based on the standard scoring criteria for RRLM. Two different scoring criteria currently are available: (1) those endorsed by the WASM/IRLSSG,3 and used in this study, in which RRLM are defined as LM occurring within 0.5 sec preceding and 0.5 sec following the end of an apnea event; and (2) those endorsed by the AASM4 that consider as RRLM any movement occurring within 0.5 sec preceding the start of an apnea event, and during 0.5 sec following the end of a breathing episode. Both these criteria are based on consensus and empiric experience and have not been addressed with analytic approaches. Preliminary evidence is available showing that leg movements very rarely occur at the beginning or during the apnea, but many follow the end of the apnea, often exceeding the time window of 0.5 sec and are not picked up by either method.30 We also performed the same aforementioned analysis in Figure 2 by using the AASM criteria4 (see supplemental material) and found less clear-cut results, with the RRLM peak extending approximately from 20-56 sec, instead of the two distinct peaks at approximately 24 and 42 sec observed with the WASM/IRLSSG criteria (Figure 2).3 If only RRLM were correctly picked up by this method, we should have observed a peak similar to that at 42 sec shown in Figure 2, which follows the distribution of intervals expected for the apnea events.12,13,25 For this reason, we have focused our attention on the WASM method14 in this study.
In conclusion, our results indicate that RRLM might represent part of a phenotypic spectrum that also includes “genuine” PLMS because they cluster clearly in the patients who also have typical PLMS not correlated with the respiratory events. It is possible to speculate that the same genetic background26 might predispose the individuals to present typical PLMS, when no other strong synchronizing mechanisms are at work, or RRLM, when the more powerful respiratory rhythm takes the lead and forces LM to synchronize with it accordingly. This might also suggest that in discussion of future scoring criteria, RRLM should not be excluded from the analysis of PLMS. Finally, for the definition of RRLM we have strictly applied the criteria set by the WASM/IRLSSG3 that, similar to those indicated by the AASM,4 are consensus based; however, our data point to a need to explore in more detail the time structure of LM, apnea, and arousals that might provide important hints for a better understanding of how these phenomena interact with each other.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was partially supported by the Italian Ministry of Health (“Ricerca Corrente”). Dr. Ferri has consulted for Merck & Co., Sapio Life, and ATES Medica Device. The other authors have indicated no financial conflicts of interest. This work was performed at the Sleep and Epilepsy Center, Neurocenter of Southern Switzerland, Civic Hospital (EOC) of Lugano, Lugano, Switzerland and the Sleep Research Centre, Department of Neurology I.C., Oasi Institute (IRCCS), Troina, Italy.
ACKNOWLEDGMENTS
This work was partially supported by the Italian Ministry of Health (“Ricerca Corrente”) and by the Swiss government (“Swiss Government scholarships for university, fine arts and music schools for foreign students”).
SUPPLEMENTAL MATERIAL
We performed the same analysis reported in Figure 2 in the article by using the American Academy of Sleep Medicine criteria1 for the identification of respiratory-related leg movements (RRLM) and found less clear-cut results with the RRLM peak extending approximately from 20 to 56 s and mimicking that of non-RRLM (NRLM).
Intermovement interval distribution histograms of the different LM activities recorded in OSAS patients with or without RRLM.
REFERENCE
- 1.Iber C, Ancoli-Israel S, Chesson AL, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
REFERENCES
- 1.Al-Alawi A, Mulgrew A, Tench E, Ryan CF. Prevalence, risk factors and impact on daytime sleepiness and hypertension of periodic leg movements with arousals in patients with obstructive sleep apnea. J Clin Sleep Med. 2006;2:281–7. [PubMed] [Google Scholar]
- 2.Iriarte J, Alegre M, Irimia P, Urriza J, Artieda J. Relevancia clinica de los movimientos periodicos de las piernas durante el sueno en pacientes con apneas obstructivas del sueno. Rev Neurol. 2000;30:101–4. [PubMed] [Google Scholar]
- 3.Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7:175–83. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Iber C, Ancoli-Israel S, Chesson AL, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, And Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 5.Seo WH, Guilleminault C. Periodic leg movement, nasal CPAP, and expiratory muscles. Chest. 2012;142:111–8. doi: 10.1378/chest.11-1563. [DOI] [PubMed] [Google Scholar]
- 6.Baran AS, Richert AC, Douglass AB, May W, Ansarin K. Change in periodic limb movement index during treatment of obstructive sleep apnea with continuous positive airway pressure. Sleep. 2003;26:717–20. doi: 10.1093/sleep/26.6.717. [DOI] [PubMed] [Google Scholar]
- 7.Fry JM, DiPhillipo MA, Pressman MR. Periodic leg movements in sleep following treatment of obstructive sleep apnea with nasal continuous positive airway pressure. Chest. 1989;96:89–91. doi: 10.1378/chest.96.1.89. [DOI] [PubMed] [Google Scholar]
- 8.Manconi M, Vitale G, Ferri R, Zucconi M, Ferini-Strambi L. Periodic leg movements in Cheyne-Stokes respiration. Eur Respir J. 2008;32:1656–62. doi: 10.1183/09031936.00163507. [DOI] [PubMed] [Google Scholar]
- 9.Chervin RD. Periodic leg movements and sleepiness in patients evaluated for sleep-disordered breathing. Am J Respir Crit Care Med. 2001;164:1454–8. doi: 10.1164/ajrccm.164.8.2011062. [DOI] [PubMed] [Google Scholar]
- 10.Vernet C, Redolfi S, Attali V, et al. Residual sleepiness in obstructive sleep apnoea: phenotype and related symptoms. Eur Respir J. 2011;38:98–105. doi: 10.1183/09031936.00040410. [DOI] [PubMed] [Google Scholar]
- 11.Yang CK, Jordan AS, White DP, Winkelman JW. Heart rate response to respiratory events with or without leg movements. Sleep. 2006;29:553–6. doi: 10.1093/sleep/29.4.553. [DOI] [PubMed] [Google Scholar]
- 12.Carelli G, Krieger J, Calvi-Gries F, Macher JP. Periodic limb movements and obstructive sleep apneas before and after continuous positive airway pressure treatment. J Sleep Res. 1999;8:211–6. doi: 10.1046/j.1365-2869.1999.00153.x. [DOI] [PubMed] [Google Scholar]
- 13.Briellmann RS, Mathis J, Bassetti C, Gugger M, Hess CW. Patterns of muscle activity in legs in sleep apnea patients before and during nCPAP therapy. Eur Neurol. 1997;38:113–8. doi: 10.1159/000113173. [DOI] [PubMed] [Google Scholar]
- 14.Ferri R, Zucconi M, Manconi M, Plazzi G, Bruni O, Ferini-Strambi L. New approaches to the study of periodic leg movements during sleep in restless legs syndrome. Sleep. 2006;29:759–69. [PubMed] [Google Scholar]
- 15.Ferri R. The time structure of leg movement activity during sleep: the theory behind the practice. Sleep Med. 2012;13:433–41. doi: 10.1016/j.sleep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 16.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 17.Manconi M, Ferri R, Feroah TR, Zucconi M, Ferini-Strambi L. Defining the boundaries of the response of sleep leg movements to a single dose of dopamine agonist. Sleep. 2008;31:1229–37. [PMC free article] [PubMed] [Google Scholar]
- 18.Manconi M, Ferri R, Zucconi M, et al. Pramipexole versus ropinirole: Polysomnographic acute effects in restless legs syndrome. Mov Disord. 2011;26:892–5. doi: 10.1002/mds.23543. [DOI] [PubMed] [Google Scholar]
- 19.Manconi M, Ferri R, Zucconi M, et al. Preferential D2 or preferential D3 dopamine agonists in restless legs syndrome. Neurology. 2011;77:110–7. doi: 10.1212/WNL.0b013e3182242d91. [DOI] [PubMed] [Google Scholar]
- 20.Manconi M, Ferri R, Zucconi M, et al. Dissociation of periodic leg movements from arousals in restless legs syndrome. Ann Neurol. 2012;71:834–44. doi: 10.1002/ana.23565. [DOI] [PubMed] [Google Scholar]
- 21.Ferri R, Manconi M, Lanuzza B, et al. Age-related changes in periodic leg movements during sleep in patients with restless legs syndrome. Sleep Med. 2008;9:790–8. doi: 10.1016/j.sleep.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Ferri R, Gschliesser V, Frauscher B, Poewe W, Hogl B. Periodic leg movements during sleep and periodic limb movement disorder in patients presenting with unexplained insomnia. Clin Neurophysiol. 2009;120:257–63. doi: 10.1016/j.clinph.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Ferri R, Zucconi M, Manconi M, et al. Different periodicity and time structure of leg movements during sleep in narcolepsy/cataplexy and restless legs syndrome. Sleep. 2006;29:1587–94. doi: 10.1093/sleep/29.12.1587. [DOI] [PubMed] [Google Scholar]
- 24.Manconi M, Ferri R, Zucconi M, Fantini ML, Plazzi G, Ferini-Strambi L. Time structure analysis of leg movements during sleep in REM sleep behavior disorder. Sleep. 2007;30:1779–85. doi: 10.1093/sleep/30.12.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charbonneau M, Marin JM, Olha A, Kimoff RJ, Levy RD, Cosio MG. Changes in obstructive sleep apnea characteristics through the night. Chest. 1994;106:1695–701. doi: 10.1378/chest.106.6.1695. [DOI] [PubMed] [Google Scholar]
- 26.Stefansson H, Rye DB, Hicks A, et al. A genetic risk factor for periodic limb movements in sleep. N Engl J Med. 2007;357:639–47. doi: 10.1056/NEJMoa072743. [DOI] [PubMed] [Google Scholar]
- 27.Winkelman JW. Periodic limb movements in sleep--endophenotype for restless legs syndrome? N Engl J Med. 2007;357:703–5. doi: 10.1056/NEJMe078129. [DOI] [PubMed] [Google Scholar]
- 28.Benedikstdottir B, Arnardottir ES, Janson C, Pack A, Juliusson S, Gislason T. Prevalence of restless legs syndrome among patients with obstructive sleep apnea before and after CPAP treatment, compared to the general population - The Icelandic Sleep Apnea Cohort (ISAC) study. Sleep. 2012;35:A265. (Abstract Supplement) [Google Scholar]
- 29.Parrino L, Thomas RJ, Smerieri A, Spaggiari MC, Del Felice A, Terzano MG. Reorganization of sleep patterns in severe OSAS under prolonged CPAP treatment. Clin Neurophysiol. 2005;116:2228–39. doi: 10.1016/j.clinph.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Fulda S, Zavalko I, Ferri R, Manconi M. Respiratory-related leg movements: What is the evidence behind the results? Sleep. 2013;36:A241–2. (Abstract Supplement) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intermovement interval distribution histograms of the different LM activities recorded in OSAS patients with or without RRLM.


