Abstract
In this article, we describe a case of bacteraemic pneumonia caused by Neisseria meningitidis serogroup Y.
A 94-year-old man sought medical care for left sided chest pain and difficulty in breathing that began 1 day before admission. He had been healthy until 4 days before admission, when sore throat, rhinorrhea, mild cough, and muscle pain. He had medical history of ischemic cardiopathy.
On physical examination, he appeared ill with respiratory distress. The respiratory rate was 60 per minute, the heart rate was 120 beats per minute, the temperature was 38.2 °C, and the blood oxygen saturation was 88% in room air. The blood pressure was 94/68 mm. The heart sounds were normal. The abdomen was soft without hepatosplenomegaly. His neck was supple without signs of meningeal irritation. On chest auscultation, coarse sounds with crackles over both bases were heard. Chest radiograph showed diffuse homogeneous infiltration in the upper lung zones and a confluent area in the right middle lobe without pleural effusion. (Fig. 1).
Fig. 1.
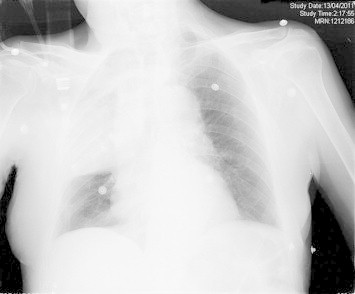
Chest radiograph showed diffuse homogeneous infiltration in the upper lung zones and a confluent area in the right middle lobe without pleural effusion.
His white blood cell (WBC) count on admission was 4300 cells/mm3 (neutrophils 90%, lymphocytes 6%) and C-reactive protein was 181 mg/dL (reference: <5 mg/dL). The hemoglobin was 14.6 g/dL, and the platelets were 165 x 103/mm3. The venous pH was 7.38, the PCO2 was 38.1 mm/Hg, and the base excess was −2.2. The lactate concentration was 2.30 mmol/L. The serum creatinine was 1.20 mg/dL, and the serum urea nitrogen was 43 mg/dL. The total protein was 6.9 g/dL. The aspartate aminotransferase was 20 U/L, and the alanine aminotransferase was 14 U/L. The total bilirubin was 0.7 mg/dL.
He was admitted in pneumology unit with a diagnosis of community pneumonia and empirical intravenous regimen of clarithromycin (500 mg/day) and ceftriaxone (2 g/day) was commenced before the microbiology results were reported.
Blood cultures taken during the patient’s febrile episode were incubated in an automated BACTEC™ FX system [Becton Dickinson, Frank*lin Lakes, NJ, USA]. Both aerobic and anaerobic bottle cultures became positive after 3 days of incubation for gram-negative diplococcus. The organism was subcultured onto sheep blood agar, chocolate agar and Brucella blood agar. The sheep blood agar and chocolate blood agar plates were incubated at 35 °C in atmosphere containing 5% CO2 for 48 h. The Brucella blood agar was incubated at 35 °C in atmosphere anaerobic for two days.
The organism isolated from blood culture at admission was oxidase, catalase and ONPG positive, and utilized glucose, maltose and lactose. This organism was identified as Neisseria meningitidis by the VITEK NHI Identification card (bioMerieux) (identification profile 10520, 99% identity) and by matrix-assisted laser desorption/ionisation time-of-flight (MALDI-TOF) mass spectrometry. The isolates are serogrouped by agglutination using commercial antisera Difco™ (Detroit, MI, USA).
Susceptibility testing was performed with the Wider® system (Fco. Soria Melguizo) and the isolate was sensitive to cefotaxime (CMI ≤0.03 μg/mL), meropenem, quinolonas, cloramfenicol and rifampicina and the susceptibility was intermediate to penicillin and ampicilin. Treatment was accordingly changed to ceftriaxone 2 g/24 h given intravenously.
The fever gradually subsided after 2 days of cefotaxime, and the patient’s general condition gradually improved. The patient was discharged after 8 days of antibiotics.
1. Discussion
N. meningitidis is a Gram-negative aerobic diplococcus, which is a normal commensal of the human nasopharynx. Meningococcal meningitis and meningococcemia are the 2 clinical syndromes with which it is traditionally associated, resulting from invasion of the local tissues into the bloodstream. It may also cause conjunctivitis, pharyngitis, pneumonia, pericarditis, septic arthritis, and urethritis.6 This organism is classified into 13 serogroups, and most meningococcal disease is caused by strains that express 1 of the 5 types of capsular polysaccharides (A, B, C, Y, and W135). N. meningitidis serogroups B and C have been responsible for the majority of invasive meningococcal disease in Europe.
In the mid-1990s, the incidence of disease due to serogroup Y increased substantially in the United States (US).1,8 During the last decade, there has also been an increase of meningococcal disease caused by serogroup Y in Canada and Colombia.9,10 In addition, some northern European countries, displayed higher proportions of disease caused by this serogroup: for example, in Norway in 2009 and 2010, the proportion was 31%; in Finland, it was 38% in 2010 and in Sweden, the proportion was of the 39%, with an incidence of 0.23 per 100,000 population during 2010.7
Meningococcal pneumonia is infrequent, is estimated to occur in <5%–15% of patients with invasive meningococcal disease, although the precise incidence is difficult to establish because of uncertainty in establishing the cause of pneumonia.2,3 Serogroup Y is more likely than other serogroups to be associated with pneumonia.3 Blood or pleural cultures that yield N. meningitidis establish the diagnosis with certainty.
Meningococcal colonization of the nasopharyngeal mucosae is a critical initial step in the pathogenesis of systemic infection. Several cell surface structures have been identified that function as adhesins in attachment of meningococci to respiratory epithelial cells. After nasopharyngeal colonization, microaspiration of upper respiratory tract secretions containing N. meningitidis probably occurs, with the subsequent development of pneumonia. Which virulence factors are operative in the production of lung infection and whether they are unique to serogroup Y meningococci are unknown. In addition, the conditions accounting for the increase in serogroup Y infections remain undefined.2
Other authors have described a predilection of serogroup Y meningococcus for causing respiratory illness, including a large outbreak of predominantly respiratory. Smilack et al. reported a military outbreak that included 12 cases of serogroup Y meningococcal disease (SYMD) among members of an army combat training unit. In this series, 5 patients had meningococcemia, 5 had meningitis, and 2 presented with primary meningococcal pneumonia.4 Subsequently, a case series of SYMD was reported in a group of US Air Force recruits in 1971–1974.6 In that series, the predominant manifestation of serogroup Y disease was respiratory; 68 (77%) of 88 patients had meningococcal pneumonia, documented by transtracheal aspirates in 94% of the cases. Only 4 (6%) of the 68 patients with pneumonia had positive blood cultures.5 Among the patients with pneumonia, the response to antibiotic therapy was prompt; 93% of the patients were afebrile within 3 days of antibiotic therapy.2 The outcome of meningococcal pneumonia when treated is generally favorable, but the diagnosis requires a high index of suspicion, testing of respiratory samples, and blood cultures.
In conclusion, we report a case of bacteraemic pneumonia caused by N. meningitidis serogroup Y with reduced susceptibility to penicillin in an adult patient.
Conflict of interest statement
All authors report no conflicts of interest relevant to this study.
References
- 1.Rosenstein N.E., Perkins B.A., Stephens D.S., Lefkowitz L., Cartter M.L., Danila R. The changing epidemiology of meningococcal disease in the United States, 1992–1996. J Infect Dis. 1999;180:1894–1901. doi: 10.1086/315158. [DOI] [PubMed] [Google Scholar]
- 2.Winstead J.M., McKinsey D.S., Tasker S. Meningococeal pneumonia: characterization and review of cases seen over the past 25 years. Clin Infect Dis. 2000;30:87–94. doi: 10.1086/313617. [DOI] [PubMed] [Google Scholar]
- 3.Rosenstein N.E., Perkins B.A., Stephens D.S., Popovic T., Hughes J.M. Meningococcal disease. N Engl J Med. 2001;344:1378–1388. doi: 10.1056/NEJM200105033441807. [DOI] [PubMed] [Google Scholar]
- 4.Smilack J.D. Group-Y meningococcal disease: twelve cases at anarmytraining center. AnnIntern Med. 1974;81:740–745. doi: 10.7326/0003-4819-81-6-740. [DOI] [PubMed] [Google Scholar]
- 5.Koppes G., Ellenbogen C., Gebhart R. Group Y meningococcal disease in United States Air Force recruits. Am J Med. 1977;62:661–666. doi: 10.1016/0002-9343(77)90867-1. [DOI] [PubMed] [Google Scholar]
- 6.Harrison L.H., Kreiner C.J., Shutt K.A., Messonnier N.E., O’Leary M., Stefonek K.R., Meningococcal High School Study Group Risk factors for meningococcal disease in students in grades 9–12. Pediatr Infect Dis J. 2008 Mar;27(3):193–199. doi: 10.1097/INF.0b013e31815c1b3a. [DOI] [PubMed] [Google Scholar]
- 7.Thulin Hedberg S., Törös B., Fredlund H., Olcén P., Mölling P. Genetic characterisation of the emerging invasive Neisseria meningitidis serogroup Y in Sweden, 2000 to 2010. Euro Surveill. 2011;16:23. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Emerging Infections Program Network; Nov 2010. Active bacterial core Surveillance (ABCs) report.http://www.cdc.gov/abcs/reportsfindings/survreports/mening09.pdf Neisseria meningitidis, 2009. Oct2010 File – 17. Available from: [Google Scholar]
- 9.Tsang R.S., Henderson A.M., Cameron M.L., Tyler S.D., Tyson S., Law D.K. Genetic and antigenic analysis of invasive serogroup Y Neisseria meningitidis isolates collected from 1999 to 2003 in Canada. J Clin Microbiol. 2007;45(6):1753–1758. doi: 10.1128/JCM.02134-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inés Agudelo C., Sanabria O.M., Ovalle M.V. Serogroup Y meningococcal disease, Colombia. Emerg Infect Dis. 2008;14(6):990–991. doi: 10.3201/eid1406.071357. [DOI] [PMC free article] [PubMed] [Google Scholar]


