Abstract
Background
The risk of urinary tract infection (UTI) is higher in diabetics compared to non-diabetics. The aetiology and the antibiotic resistance of uropathogens have been changing over the past years. Hence the study was undertaken to determine if there are differences in clinical and microbiological features of UTI between diabetic and non-diabetic subjects, to study the influence of diabetes mellitus on the uropathogens and antibiotic sensitivity pattern in patients with UTI.
Method
A total of 181 diabetics (83 males and 98 females) and 124 non-diabetic subjects (52 males and 72 females) with culture positive UTI were studied. Patients with negative urine culture (n= 64), those diagnosed and treated outside (n= 83) and not willing to participate in the study (n= 24) were excluded.
Results
Almost 30 per cent of the patients (both diabetics and nondiabetics) presented with asymptomatic bacteriuria and the prevalence of pyelonephritis was significantly higher (p= 0.04) in diabetics compared to non-diabetic patients. The majority of the diabetics with UTI (87.14 per cent) had glycosylated haemoglobin (HbA1c) > 6.5 per cent with p < 0.001. The isolation rate of Escherichia coli (E. coli) from urine culture was higher (64.6 per cent) among diabetic patients followed by Klebsiella (12.1 per cent) and Enterococcus (9.9 per cent). The prevalence of extendedspectrum beta-lactamase (ESBL) producing E.coli was significantly higher in diabetics (p= 0.001) compared to nondiabetics. E.coli showed maximum sensitivity to carbapenems in both diabetic and non-diabetic subjects and least susceptibility to ampicillin.
Conclusion
The prevalence of pyelonephritis is significantly higher in diabetics than in non-diabetic subjects, with E. coli being the most common isolate. Elevated glycosylated hemoglobin (HbA1c) predisposes diabetics to UTI. Investigation of bacteriuria in diabetic patients for urinary tract infection is important for treatment and prevention of renal complications.
Keywords: Urinary tract infection, Asymptomatic bacteriuria, Uropathogens, Diabetic patients, E. coli
What this study adds:
UTI is a significant problem both among diabetics and non-diabetics
E. coli is the most frequent pathogen responsible for UTI and recurrent UTI among both diabetics and non-diabetics followed by Klebsiella and Enterococcus. More than 75 per cent of the organisms including including ESBL E. coli, Pseudomonas and Acinetobacter were sensitive to amikacin and meropenem.
The widespread use of antimicrobial agents leads to emergence of drug resistant organisms. Since the pattern of bacterial resistance is constantly changing over years, it is important to monitor the antibiotic susceptibility patterns of isolated organisms to ensure rational use of antibiotics for empirical and definitive treatment of urinary tract infections in the vulnerable group.
Background
Diabetics are more prone to infections than their nondiabetic counterparts. The urinary tract is the most common site of infection in diabetic patients. Most of the urinary tract infections (UTIs) in diabetic patients are relatively asymptomatic, which can lead to severe kidney damage and renal failure. Bacteriuria is more common in diabetics than in non-diabetics due to a combination of host and local risk factors.1
Disturbances (low complement factor 4, decreased cytokine response) in humoral innate immunity have been described in diabetic patients.2 However, the clinical relevance of these findings is not clear. Concerning cellular innate immunity, most studies show decreased function in diabetic polymorphonuclear cells and monocytes/macrophages compared to controls. Improved control of the diabetes mellitus (DM) can lead to an improvement in these cellular functions. As well, some microorganisms become more virulent in a high glucose environment.2 Therefore, screening for UTI in diabetic patients is very important to enable bacteruria to be properly treated, and prevent the development of renal complications of diabetes and eventually severe renal damage and failure.3 However, controversies exist with respect to incidence, prevalence and microbiological features of UTI between diabetic and non-diabetic patients.4 Hence the study was planned to compare clinical, microbiological and predisposing features of UTI in diabetics and non-diabetics. The aim of this study was to investigate if differences exist in the clinical and microbiological characteristics of UTI between diabetic and non-diabetic patients and to study the influence of diabetes mellitus on the spectrum of uropathogens and antimicrobial resistance pattern in patients with UTI.
Method
This was a prospective study conducted at the Department of Medicine at a tertiary care hospital in Karnataka. The study was carried out from October 2010 to June 2012. A total of 476 patients were screened, of which 305 patients were included in the study. The study included 181 diabetic (83 males and 98 females) and 124 non-diabetic patients (52 males and 72 females) with culture positive UTIs. Patients with negative urine culture (n= 64), those diagnosed and treated outside (n= 83), not willing to participate in the study (n= 24) or with an age < 18 years were excluded. The following data including age, sex, occupation and symptomatology were taken and clinical examination was done. All proven diabetics with fasting venous glucose > 126mg/dl and postprandial (2h) venous glucose > 200mg/dl were included in the study irrespective of reason for admission. Patients with a history of diabetes and those who were on treatment for the same were also eligible for admission. Controls consisted of patients admitted in hospital with comparable age and sex with no history of diabetes and fasting blood sugar < 110mg/dl.
The laboratory tests included complete blood picture, renal and liver function test and urine microscopy including culture. For urine microscopy, 5ml of clean catch midstream urine was centrifuged at 3000 rpm for five minutes and centrifuge was viewed under microscope and more than five WBC per high power field was considered significant. A fasting sugar, postprandial sugar and HbA1c were done for all diabetics. Data was analysed using statistical package SPSS version 16. The percentages in different categories were compared using Chi square test and means were compared using Student’s t-test. A p-value less than 0.05 were considered significant.
Results
The mean age among diabetic and non-diabetic patients was 60.2 ± 13.76 years and 53.47 ± 18.56 years. Duration of diabetes was less than one year in 33 (18 per cent) patients, 1 to 10 years in 109 (60 per cent) patients and greater than 10 years in 39 (22 per cent) patients. Of 181 patients, 46 per cent (83) of the diabetics were on oral hypoglycemic agents (OHA’s) alone, 38 per cent (68) were on insulin and 16 per cent (30) were on both insulin and OHA treatment.
Table 1 shows the clinical characteristics of the groups. There was no significant statistical difference in clinical symptoms between diabetic and non-diabetic subjects calculated by Chi-square test. Although fever was the most common presenting symptom, almost 30 per cent of the patients (both diabetics and non-diabetics) did not present with any urinary symptoms as shown in Figure 1.
Table 1. Clinical characteristics.
| Symptoms | DM | NON-DM | p-value |
|---|---|---|---|
| Fever | 104 (57.4%) | 81 (65.3%) | 0.94 |
| Dysuria | 75 (41.4%) | 55(44.3%) | 0.83 |
| Increased frequency | 43 (23.7%) | 38 (30.8%) | 0.52 |
| Abdominal pain | 35 (19.3%) | 34 (27.4%) | 0.84 |
| Vomiting | 44 (24.3%) | 23 (18.3%) | 0.24 |
| Haematuria | 8 (4.4%) | 4 (3.2%) | - |
| Pyuria | 7 (3.8%) | 3 (2.4%) | - |
| Incontinence | 26 (14.4%) | 15 (12.09%) | 0.18 |
| Retention | 5 (2.7%) | 5 (4.03%) | - |
Figure 1. Asymptomatic bacteriuria.
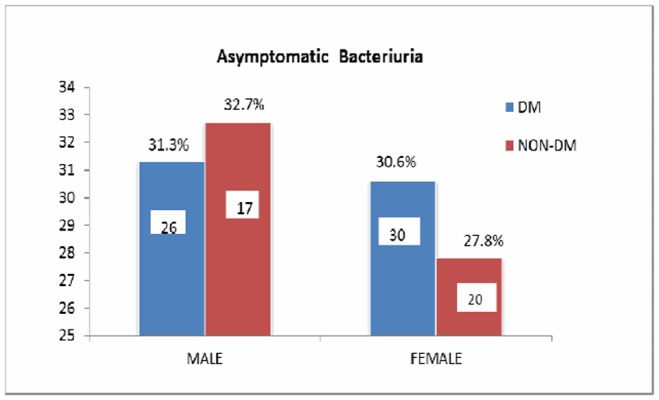
The prevalence of pyelonephritis is significantly higher (p= 0.04) in diabetics (9.4 per cent compared with non-diabetic patients 3.2 per cent (Table 2). The most common organism isolated being E. coli (Table 3).
Table 2. Pyelonephritis in diabetics.
| DM (n=181) | NON-DM (n=124) | |
|---|---|---|
| Asymptomatic bacteriuria | 56 | 37 |
| Pyelonephritis | 17 | 4 |
Table 3. Microorganisms causing pyelonephritis.
| DM | NON-DM | |
|---|---|---|
| E. coli | 9 | 2 |
| Klebsiella | 2 | 0 |
| Enterococcus | 3 | 0 |
| Pseudomonas | 1 | 2 |
| Proteus | 1 | 0 |
| Candida | 1 | 0 |
Benign prostatic hypertrophy (BPH) was the most common predisposing factor in both diabetic (38.5 per cent) and nondiabetic males (40.3 per cent) followed by catheterisation in diabetic (37.3 per cent) and non-diabetic (44.2 per cent) subjects (Table 4). The most common predisposing condition for UTI in females was the presence of indwelling catheter in diabetic (33.6 per cent) and non-diabetic (30.5 per cent) (Table 4). There was no significant difference in the p-value (0.83) among both groups. The past history of UTI was observed in 27 per cent of diabetics and 18 per cent of non-diabetics. However this difference was also not statistically significant.
Table 4. Predisposing conditions for UTI.
| DM | NON-DM | |||
|---|---|---|---|---|
| M | F | M | F | |
| BPH | 38.5% | - | 40.3% | - |
| Indwelling catheter | 37.3% | 33.6% | 44.2% | 30.5% |
| Hydroureternephrosis | 9.6% | 7.1% | 15.3% | 8.3% |
| Stricture urethra | 8.4% | - | 9.6% | - |
| Phimosis | 3.6% | - | 3.8% | - |
| Calculi | 6% | 3% | 7.6% | 1.3% |
| Recent genitourinary surgery/ instrumentation | 7.2% | 7.6% | - | |
| Balanoposthitis | 1.2% | - | 0 | - |
| Neurogenic bladder | 3.6% | - | 0 | - |
| Meatal stenosis | - | 2% | - | 1.3% |
| Gynaecological disorders | - | 6.1% | - | 11.1% |
| Pregnancy | - | 13.2% | - | 8.3% |
Among the complications, Acute Kidney Injury (AKI) was most common followed by recurrent UTI. Recurrent UTI was noted in 26 of 181 diabetics and 13 of 124 non-diabetic subjects. The prevalence of recurrent UTI was higher in diabetics (14.4 per cent) compared to non-diabetics (10.5 per cent); however difference was not statistically significant. The most common uropathogen among these patients was E. coli. More than 50 per cent of patients with recurrent UTI had HbA1c ≥ 8.0 (poorly controlled DM). Mean HbA1c in diabetics with recurrent UTI was 9.26 ± 3.83 (i.e. > 8.0). Renal papillary necrosis was observed in two cases of Candidial septicaemia.
The micro-organisms isolated from the urine cultures are listed in Table 5. The most common organism isolated among both diabetics and non-diabetics was E. coli. When patients with indwelling catheter were considered separately, the isolation rates of different uropathogens significantly differed only for pseudomonas in diabetic and non-diabetic groups (3.1 per cent vs 17.8 per cent) (p< 0.05). We observed a higher isolation rate of Pseudomonas spp. in non-diabetic males than that in diabetic males (15.4 per cent vs 3.6 per cent) (p < 0.05). Acinetobacter spp was isolated from urine cultures from three diabetic subjects, out of which twp patients also had positive blood cultures for the same organism and one case had endotracheal aspirate growth for Acinetobacter spp. Candida spp. was isolated from both blood and urine cultures in five diabetic patients. The prevalence of ESBL E. coli was significantly higher in diabetics (p= 0.001) compared to non-diabetics.
Table 5. Organisms isolated from urine cultures.
| Organisms | DM | NON-DM | p-value |
|---|---|---|---|
| E. coli | 117 | 73 | NS |
| Klebsiella | 22 | 18 | NS |
| Enterococcus | 18 | 10 | NS |
| Pseudomonas | 3 | 15 | < 0.05 |
| Acinetobacter | 3 | 0 | - |
| Citrobacter | 3 | 2 | - |
| Proteus | 3 | 1 | - |
| Coag. Negative Staph | 3 | 4 | - |
| Coag. Positive Staph | 4 | 1 | - |
| Candida | 5 | 0 | - |
The antimicrobial resistance pattern was similar in both groups with maximum sensitivity to meropenem and least susceptibility to ampicillin as noted in Table 6. Aminoglycosides showed a better sensitivity profile than cefoperazone-sulbactum in both diabetics and nondiabetics; however the number of patients were too small to draw any conclusions from the above mentioned observation. Enterococcus showed maximum susceptibility to linidazole, teicoplanin and vancomycin. Of the five patients with coagulase positive staphylococcus, two cases were MRSA isolates which were sensitive to vancomycin and linidazole.
Table 6. Comparison of antimicrobial susceptibility.
| E. coli sensitivity | Klebsiella sensitivity | Pseudomonas sensitivity | ||||
|---|---|---|---|---|---|---|
| DM | NON-DM | DM | NON-DM | DM | NONDM | |
| Amikacin | 80.7% | 79.3% | 88.9% | 91.3% | 93.8% | 75% |
| Ampicilin | 16.7% | 17% | 11.1% | 17.4% | 6.2% | 12.5% |
| Augmentin | 42.6% | 38.3% | 55.6% | 47.8% | 18.8% | 25% |
| Aztreonam | 22.2% | 22.6% | 38.2% | 36.4% | 25% | 37.5% |
| Cefotaxime | 44.4% | 36.4% | 38.9% | 43.5% | 25% | 12.5% |
| Cefepime | 48.9% | 36.4% | 54% | 49.5% | 34% | 42.6% |
| Gentamycin | 68.5% | 67% | 72.2% | 65.2% | 56.2% | 67.5% |
| Cefoperazonesulbactum | 85.0% | 80.4% | 87.5% | 94.7% | 68.8% | 62.5% |
| Meropenem | 93.8% | 95.2% | 95.8% | 100% | 87.5% | 87.5% |
| Netilmycin | 75.8% | 78.5% | 82.2% | 89.4% | 78.8% | 100% |
| Norfloxacin | 25% | 34% | 33.3% | 34.8% | 16.2% | 37.5% |
| Piperacillin- Tazobactum | 68.5% | 73.6% | 88.9% | 73.9% | 81.2% | 62.5% |
| Cotrimoxazole | 38.9% | 30.2% | 38.9% | 30.4% | 18.8% | 12.5% |
| Ceftriaxone | 50% | 44.9% | 48.3% | 43.6% | 22.5% | 28% |
Discussion
In the present study, there was no significant correlation between the age of patient and the incidence of UTI in both diabetic and non-diabetic patients. Bonadio M et al. (2006) also made a similar observation in his study (73.7 years in diabetics vs 72.7 years in non-diabetic subjects).5
Bahl AL et al. (1970) found significant correlation between duration of diabetes and the prevalence of bacteriuria.6 The prevalence of bacteriuria increased 1.9 fold for every 10 years of diabetes duration. This is probably due to higher prevalence of autonomic neuropathy and subsequent incomplete bladder emptying in longstanding diabetes.7 However, such a correlation was not observed in our study. Zhanel GG et al. (1995) noted that the prevalence of UTI was significantly higher among patients on oral hypoglycaemic agents.8 There was no correlation between type of treatment and the prevalence of UTI in the present study.
Fever was the most common symptom associated with UTI in both diabetics and non-diabetics. So, the presence of fever should prompt a look at the urinary tract as a possible source of infection. However; there was no significant difference in the clinical symptoms among both groups as shown in Table 1. In the present study, no significant difference was found in the prevalence of asymptomatic bacteriuria (ASB) in females (diabetics 0.6 per cent vs. nondiabetics 27.8 per cent) and males (diabetics 31.3 per cent vs. non-diabetics 32.7 per cent). This is in agreement with the study conducted by Bonadio M et al. (2006) (diabetic females 14.9 per cent vs. non-diabetic females 13.1 per cent) and (diabetic males 12.76 per cent vs non-diabetic males 11.4 per cent).5 However in the study conducted by Geerlings SE et al. (2000) the prevalence of asymptomatic bacteriuria was higher in diabetic women when compared to non-diabetic women (26 per cent in diabetic subjects and 6 per cent in controls).9
Bladder outlet obstruction due to BPH or urethral stricture was the predisposing factor in almost 40 per cent of males with UTI in this series. The presence of underlying autonomic neuropathy in these patients was not investigated. Most of the diabetic patients developing UTI in our study had long standing DM (> 5 years). Hence, probably these patients might require more intensive screening for the presence of bacteriuria and UTI.
The mean HbA1c level of the diabetic patients at the time of admission was 8.42 per cent ± 2.8 SD in our study compared with Bonadio M et al. (2006) (the mean HbA1c level being 7.8 per cent ± 1.6 SD).5 Majority of the diabetics with UTI (87.14 per cent ) had HbA1c > 6.5 per cent with p < 0.001. A high proportion of patients (88.8 per cent) with HbA1c < 6.5 and UTI had other underlying factors such as bladder outlet obstruction or indwelling catheter which predisposed them to UTI. Thus the occurrence of UTI in diabetics seems to be related to the glycaemic control in the recent (weeks to months). Schmitt JK et al. (1986) analysed the correlation between asymptomatic bacteriuria and HbA1c and found no statistically significant association between the degree of glycemic control and UTI. A higher incidence of elevated blood glucose levels was observed in patients with UTI; but did not attribute the elevated blood glucose to be a predisposing factor for UTI.10
Tseng CC et al. (2002) noted that a HbA1c > 8.1 per cent was associated with an increased risk for UTI.11 Our study supports the findings of Tseng CC et al. (2002), who concluded that patients with HbA1c > 8.1 per cent have a higher prevalence of upper UTI. The presence of HbA1c < 6.5 per cent significantly (p= 0.026) decreased the risk of UTI irrespective of whether there was underlying predisposing factor or not. In those patients of UTI with HbA1c < 6.5 per cent, almost 90 per cent of the patients had underlying predisposing factors such as bladder outlet obstruction or indwelling catheter. Thus, achieving an HbA1c < 6.5 per cent particularly seems to protect those diabetics from UTI who do not have an underlying predisposing factor.
In the study conducted by Gorter KJ et al. (2010) relapses and re-infections were reported in 7.1 per cent and 15.9 per cent of diabetic women versus 2.0 per cent and 4.1 per cent of non-diabetic women. He concluded that there was an independent higher risk of recurrent UTI in women with diabetes compared with women without diabetes (Odds ratio 2.0; 95 per cent Confidence interval 1.4–2.9).12
E. coli was the most frequent uropathogen isolated, responsible for UTI in 60.2 per cent and 65.3 per cent of diabetic males and females and 50 per cent & 51.4 per cent of non-diabetic males & females. In the study conducted by Bonadio M et al. (2006) the isolation rates of E. coli were: diabetics (males 32.5 per cent vs females 54.1 per cent) and non-diabetics (males 31.4 per cent vs 58.2 per cent).5 The prevalence of ESBL E. coli was significantly higher in diabetics (78.6 per cent) than in non-diabetics (45.2 per cent) and isolation rates of ESBL E. coli in diabetics (47.8 per cent) vs non-diabetics were also found to be higher in this study compared to study conducted by Saber MH et al. (2010) in (9.1 per cent).13 In the present study, we observed that the spectrum of uropathogens and antimicrobial resistance patterns among patients with catheter associated UTI were different from those observed in other hospitals. This may depend on the different policy of antibiotics used in the various hospitals and the inclusion of higher number of nosocomial UTIs in our study.
Fungal UTI among diabetic population are more common in patients with prolonged hospital stay, catheterisation and prolonged parenteral antibiotic use.14 In the present study five patients had UTI due to Candida. These patients had other factors predisposing to UTI and/or prolonged hospital stay.
Regarding the antimicrobial resistance profile of the uropathogens, we observed that the isolated E. coli strains were resistant at similar rates to ampicillin, cotrimoxazole, norfloxacin and cephalosporins (Table 6) in diabetic and non-diabetic patients which are in comparison with Bonadio M et al. (2006).5 Considering the antimicrobial susceptibility, E. coli showed an increased sensitivity to carbapenems in both diabetics (93.8 per cent) and non-diabetics (95.1 per cent) and decreased susceptibility to ampicillin (diabetics 16.7 per cent vs non-diabetics 17 per cent). This is comparable to Saber MH et al. (2010) who demonstrated that E. coli sensitivity to carbapenems was 100 per cent in both diabetic and non-diabetic subjects.13
Conclusion
The prevalence of pyelonephritis is significantly higher in diabetics than in non-diabetic subjects. Elevated HbA1c correlates with occurrence of UTI and the predisposition of the diabetic to UTI depends on the degree of glycaemic control over a period of weeks to months. Achieving an HbA1c < 6.5 per cent appears to protect those diabetics who do not have other underlying predisposing factors for UTI. An HbA1c > 8.0 per cent in patients with diabetes mellitus increases the chance of developing UTI and its recurrence. E. coli is the most frequent pathogen responsible for UTI and recurrent UTI among both diabetics and non-diabetics followed by Klebsiella and Enterococcus. Investigation of bacteriuria in diabetic patients for urinary tract infection is important for treatment and prevention of the development of renal complications.
Footnotes
PEER REVIEW
Not commissioned. Externally peer reviewed
CONFLICTS OF INTEREST
The authors declare that they have no competing interests.
ETHICS COMMITTEE APPROVAL
IEC 181/2010, Ethics Committee, Kasturba Hospital, Manipal.
Please cite this paper as: Aswani SM, Chandrashekar UK, Shivashankara KN, Pruthvi BC. Clinical profile of urinary tract infections in diabetics and non-diabetics. AMJ 2014, 7, 1, 29-34. http//dx.doi.org/10.4066/AMJ.2014.1906
References
- 1.Patterson JE, Andriole VT. Bacterial urinary tract infections in diabetes. Infect Dis Clin North Am. 1997;11(3):735–50. doi: 10.1016/s0891-5520(05)70383-4. [DOI] [PubMed] [Google Scholar]
- 2.Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus. FEMS Immunol Med Microbiol. 1999;26:259–65. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 3.Kunin CM. 4th ed. Philadelphia, PA: Lea and Febiger; 1987. Detection, prevention and management of urinary tract infections. [Google Scholar]
- 4.Brauner A, Flodin U, Hylander B, Ostenson CG. Bacteriuria, bacterial virulence and host factors in diabetic patients. Diabet Med. 1993;10(6):550–4. doi: 10.1111/j.1464-5491.1993.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 5.Bonadio M, Costarelli S, Morelli G, Tartaglia T. The influence of diabetes mellitus on the spectrum of uropathogens and the antimicrobial resistance in the elderly adult patients with urinary tract infection. BMC Infect Dis. 2006;6:54. doi: 10.1186/1471-2334-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bahl AL, Chugh RN, Sharma KB. Asymptomatic bacteremia in diabetics attending a diabetic clinic. Indian J Med Sci. 1970;24:1–6. [PubMed] [Google Scholar]
- 7.Keane EM, Boyko EJ, Reller LB, Hamman RF. Prevalence of asymptomatic bacteriuria in subjects with NIDDM in San Luis Valley of Colorado. Diabetes Care. 1988;11:708–12. doi: 10.2337/diacare.11.9.708. [DOI] [PubMed] [Google Scholar]
- 8.Zhanel GG, Nicolle LE, Harding GK. Prevalence of asymptomatic bacteriuria and associated host factors in women with diabetes mellitus. The Manitoba Diabetic Urinary Infection Study Group. Clin Infect Dis. 1995;21:316–22. doi: 10.1093/clinids/21.2.316. [DOI] [PubMed] [Google Scholar]
- 9.Geerlings SE, Stolk RP, Camps MJ, Netten PM, Hoekstra JB, Bouter KP, Bravenboer B, Collet JT, Jansz AR, Hoepelman AI. Asymptomatic bacteriuria may be considered a complication in women with diabetes. Diabetes Mellitus Women Asymptomatic Bacteriuria Utrecht Study Group. Diabetes Care. 2000;23(6):744–9. doi: 10.2337/diacare.23.6.744. [DOI] [PubMed] [Google Scholar]
- 10.Schmitt JK, Fawcett CJ, Gullickson G. Asymptomatic bacteriuria and hemoglobin A1. Diabetes Care. 1986;9(5):518–20. doi: 10.2337/diacare.9.5.518. [DOI] [PubMed] [Google Scholar]
- 11.Tseng CC, Wu JJ, Liu HL, Sung JM, Huang JJ. Roles of host and bacterial virulence factors in the development of upper urinary tract infection caused by Escherichia coli. Am J Kidney Dis. 2002;39(4):744–52. doi: 10.1053/ajkd.2002.32992. [DOI] [PubMed] [Google Scholar]
- 12.Gorter KJ, Hak E, Zuithoff NP, Hoepelman AI, Rutten GE. Risk of recurrent acute lower urinary tract infections and prescription pattern of antibiotics in women with and without diabetes in primary care. Fam Pract. 2010;27(4):379–85. doi: 10.1093/fampra/cmq026. [DOI] [PubMed] [Google Scholar]
- 13.Saber MH, Barai L, Haq JA, Jilani MSA, Begum J. The pattern of organism causing urinary tract infection in diabetic and non-diabetic patients in Bangladesh. Bangladesh J Med Microbiol. 2010;4(1):6–8. [Google Scholar]
- 14.Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med. 1999;341(25):1906–12. doi: 10.1056/NEJM199912163412507. [DOI] [PubMed] [Google Scholar]


