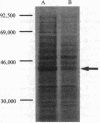
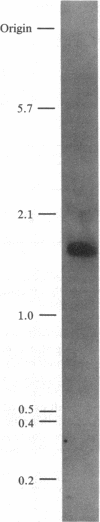
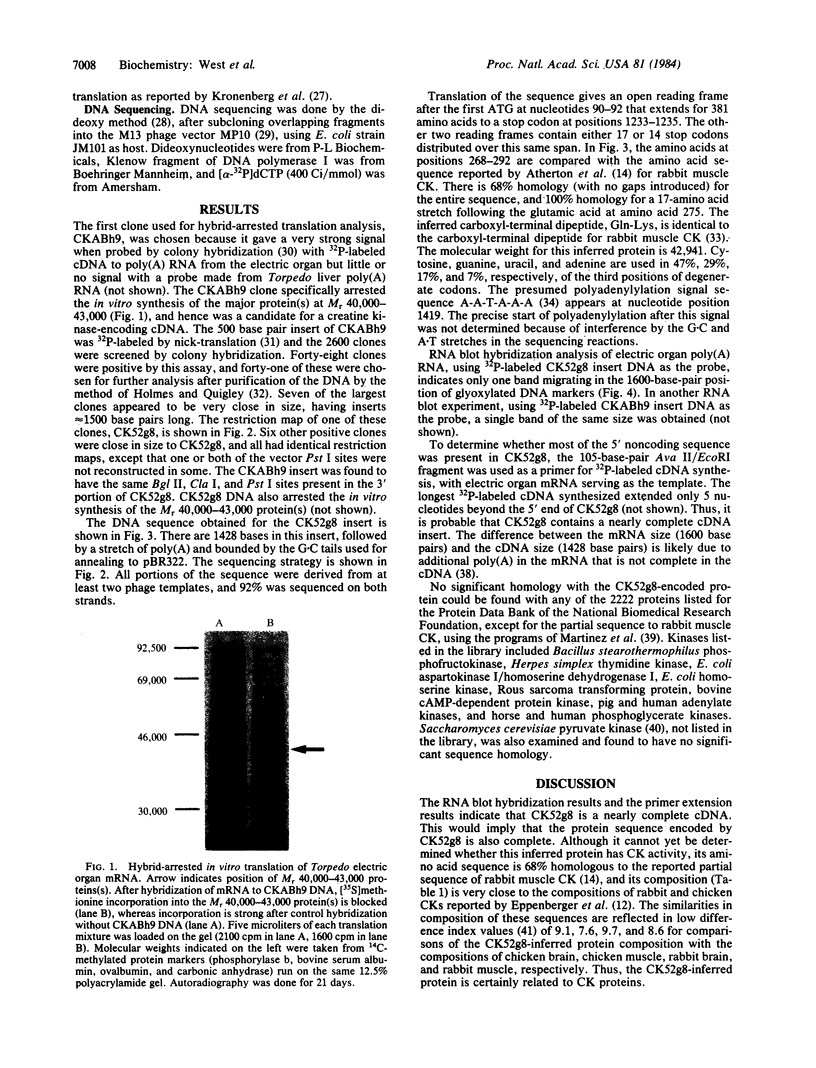
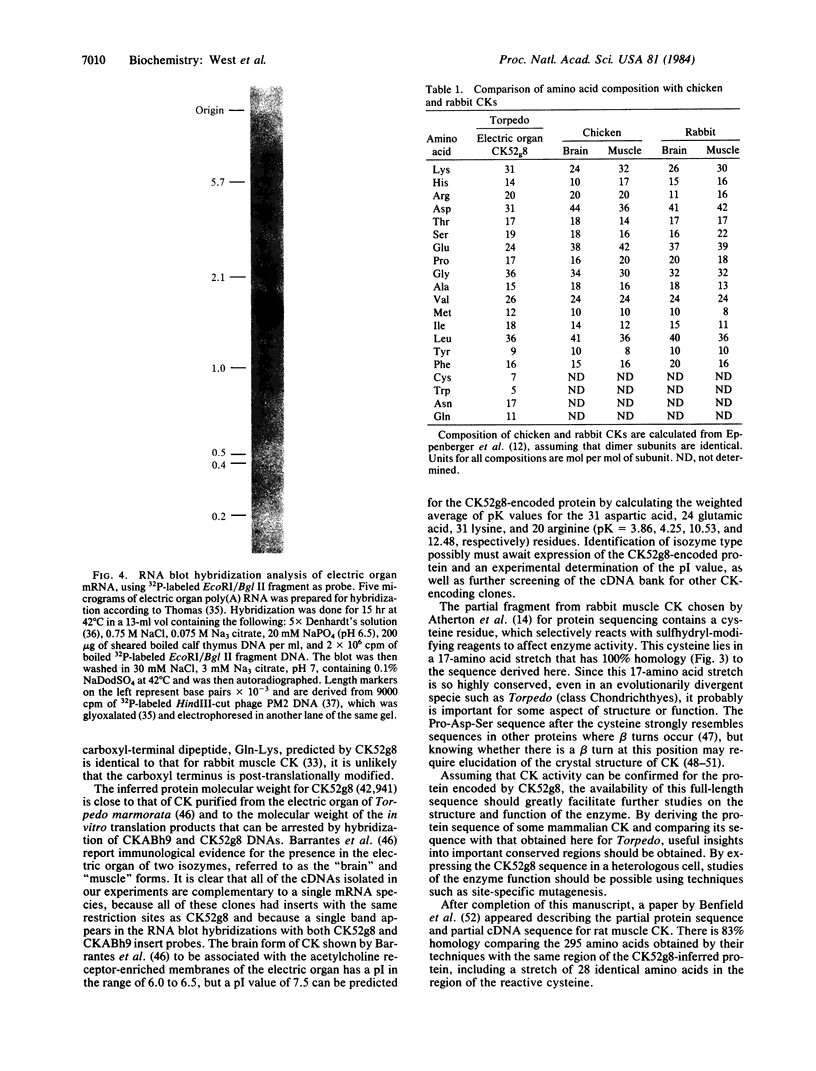
Abstract
Creatine kinase (ATP creatine N-phosphotransferase, EC 2.7.3.2) is important in the maintenance of ATP levels in high energy-requiring tissues such as muscle and brain. A complete understanding of its function requires knowledge of its amino acid sequence. To obtain cDNA clones encoding creatine kinase sequences, a cDNA bank was constructed using mRNA from the electric organ of Torpedo californica and was screened by comparing differential colony hybridization of electric organ and liver-derived 32P-labeled cDNAs. Cloned DNAs have been isolated that can arrest the abundant synthesis of Mr 40,000-43,000 material seen after in vitro translation of electric organ mRNA. One of the clones, CK52g8, was sequenced by the dideoxy M13 method and was found to encode a Mr 42,941 protein, which is 68% homologous to a known partial sequence of rabbit muscle creatine kinase and which has a composition similar to creatine kinases from chicken and rabbit tissues. By contrast, no significant homology was found with the known sequences of kinases that use other substrates. RNA blot hybridization analysis indicated that CK52g8 is complementary to a 1600-base-pair mRNA. Primer extension analysis indicated that CK52g8 is only 5 nucleotides short of a full-length cDNA, implying that it encodes a complete protein sequence. The availability of this complete sequence should be useful in further studies of creatine kinase structure and function using techniques such as site-specific mutagenesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherton R. S., Laws J. F., Miles B. J., Thomson A. R. Brain adenosine 5'-triphosphate-creatine phosphotransferase. Biochem J. 1970 Dec;120(3):589–600. doi: 10.1042/bj1200589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrantes F. J., Mieskes G., Wallimann T. Creatine kinase activity in the Torpedo electrocyte and in the nonreceptor, peripheral v proteins from acetylcholine receptor-rich membranes. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5440–5444. doi: 10.1073/pnas.80.17.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessman S. P., Geiger P. J. Transport of energy in muscle: the phosphorylcreatine shuttle. Science. 1981 Jan 30;211(4481):448–452. doi: 10.1126/science.6450446. [DOI] [PubMed] [Google Scholar]
- Borders C. L., Jr, Riordan J. F. An essential arginyl residue at the nucleotide binding site of creatine kinase. Biochemistry. 1975 Oct 21;14(21):4699–4704. doi: 10.1021/bi00692a021. [DOI] [PubMed] [Google Scholar]
- Brawerman G. The Role of the poly(A) sequence in mammalian messenger RNA. CRC Crit Rev Biochem. 1981;10(1):1–38. doi: 10.3109/10409238109114634. [DOI] [PubMed] [Google Scholar]
- Burgess A. N., Liddell J. M., Cook W., Tweedlie R. M., Swan I. D. Creatine kinase. A new crystal form providing evidence of subunit structural homogeneity. J Mol Biol. 1978 Aug 25;123(4):691–695. doi: 10.1016/0022-2836(78)90213-9. [DOI] [PubMed] [Google Scholar]
- Burke R. L., Tekamp-Olson P., Najarian R. The isolation, characterization, and sequence of the pyruvate kinase gene of Saccharomyces cerevisiae. J Biol Chem. 1983 Feb 25;258(4):2193–2201. [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Cook P. F., Kenyon G. L., Cleland W. W. Use of pH studies to elucidate the catalytic mechanism of rabbit muscle creatine kinase. Biochemistry. 1981 Mar 3;20(5):1204–1210. doi: 10.1021/bi00508a023. [DOI] [PubMed] [Google Scholar]
- Cooke N. E., Coit D., Weiner R. I., Baxter J. D., Martial J. A. Structure of cloned DNA complementary to rat prolactin messenger RNA. J Biol Chem. 1980 Jul 10;255(13):6502–6510. [PubMed] [Google Scholar]
- Duguid J. R., Raftery M. A. Fractionation and partial characterization of membrane particles from Torpedo californica electroplax. Biochemistry. 1973 Sep 11;12(19):3593–3597. doi: 10.1021/bi00743a003. [DOI] [PubMed] [Google Scholar]
- Eppenberger H. M., Dawson D. M., Kaplan N. O. The comparative enzymology of creatine kinases. I. Isolation and characterization from chicken and rabbit tissues. J Biol Chem. 1967 Jan 25;242(2):204–209. [PubMed] [Google Scholar]
- Gergen J. P., Stern R. H., Wensink P. C. Filter replicas and permanent collections of recombinant DNA plasmids. Nucleic Acids Res. 1979 Dec 20;7(8):2115–2136. doi: 10.1093/nar/7.8.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland G. L., Sjölin L., Olsson G. Crystallization and preliminary X-ray diffraction data of two crystal forms of bovine heart creatine kinase. J Mol Biol. 1983 Nov 5;170(3):791–793. doi: 10.1016/s0022-2836(83)80132-6. [DOI] [PubMed] [Google Scholar]
- Grossman S. H. Interaction of creatine kinase from monkey brain with substrate: analysis of kinetics and fluorescence polarization. J Neurochem. 1983 Sep;41(3):729–736. doi: 10.1111/j.1471-4159.1983.tb04801.x. [DOI] [PubMed] [Google Scholar]
- Hammes G. G., Hurst J. K. Relaxation spectra of adenosine triphosphate-creatine phosphotransferase. Biochemistry. 1969 Mar;8(3):1083–1094. doi: 10.1021/bi00831a040. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Jacobs H. K., Kuby S. A. Studies on adenosine triphosphate transphosphorylases. IX. Kinetic properties of the crystalline adenosine triphosphate-creatine transphosphorylase from calf brain. J Biol Chem. 1970 Jul 10;245(13):3305–3314. [PubMed] [Google Scholar]
- James T. L. Binding of adenosine 5'-diphosphate to creatine kinase. An investigation using intermolecular nuclear Overhauser effect measurements. Biochemistry. 1976 Oct 19;15(21):4724–4730. doi: 10.1021/bi00666a029. [DOI] [PubMed] [Google Scholar]
- James T. L., Cohn M. The role of the lysyl residue at the active site of creatine kinase. Nuclear Overhauser effect studies. J Biol Chem. 1974 Apr 25;249(8):2599–2604. [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations close to the AUG initiator codon affect the efficiency of translation of rat preproinsulin in vivo. Nature. 1984 Mar 15;308(5956):241–246. doi: 10.1038/308241a0. [DOI] [PubMed] [Google Scholar]
- Kronenberg H. M., McDevitt B. E., Majzoub J. A., Nathans J., Sharp P. A., Potts J. T., Jr, Rich A. Cloning and nucleotide sequence of DNA coding for bovine preproparathyroid hormone. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4981–4985. doi: 10.1073/pnas.76.10.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Land H., Grez M., Hauser H., Lindenmaier W., Schütz G. 5'-Terminal sequences of eucaryotic mRNA can be cloned with high efficiency. Nucleic Acids Res. 1981 May 25;9(10):2251–2266. doi: 10.1093/nar/9.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson A., Jr A preliminary crystallographic investigation of rabbit muscle creatine kinase. J Mol Biol. 1973 Nov 25;81(1):83–86. doi: 10.1016/0022-2836(73)90249-0. [DOI] [PubMed] [Google Scholar]
- Mendez B., Valenzuela P., Martial J. A., Baxter J. D. Cell-free synthesis of acetylcholine receptor polypeptides. Science. 1980 Aug 8;209(4457):695–697. doi: 10.1126/science.7394526. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Metzger H., Shapiro M. B., Mosimann J. E., Vinton J. E. Assessment of compositional relatedness between proteins. Nature. 1968 Sep 14;219(5159):1166–1168. doi: 10.1038/2191166a0. [DOI] [PubMed] [Google Scholar]
- Nonessentiality of the active sulfhydryl group of rabbit muscle creatine kinase. J Biol Chem. 1974 May 25;249(10):3317–3318. [PubMed] [Google Scholar]
- OLSON O. E., KUBY S. A. STUDIES ON ADENOSINE TRIPHOSPHATE-ADENOSINE TRIPHOSPHATE TRANSPHOSPHORYLASES. V. CARBOXYL-TERMINAL SEQUENCES OF ADENOSINE TRIPHOSPHATE-CREATINE TRANSPHOSPHORYLASE AND OF ADENOSINE 5'-PHOSPHATE TRANSPHOSPHORYLASE (MYOKINASE). J Biol Chem. 1964 Feb;239:460–467. [PubMed] [Google Scholar]
- Paterson B. M., Roberts B. E., Kuff E. L. Structural gene identification and mapping by DNA-mRNA hybrid-arrested cell-free translation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4370–4374. doi: 10.1073/pnas.74.10.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg U. B., Kunz G., Frischauf A., Lehrach H., Mähr R., Eppenberger H. M., Perriard J. C. Molecular cloning and expression during myogenesis of sequences coding for M-creatine kinase. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6589–6592. doi: 10.1073/pnas.79.21.6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinfest C. W., Kwiatkowski R. W., Dottin R. P. Molecular cloning of a DNA sequence complementary to creatine kinase M mRNA from chickens. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4997–5000. doi: 10.1073/pnas.79.16.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeck R. E., Gebhardt C. Physical map of PM2 DNA. Hoppe Seylers Z Physiol Chem. 1979 Apr;360(4):529–532. doi: 10.1515/bchm2.1979.360.1.529. [DOI] [PubMed] [Google Scholar]
- Takasawa T., Fukushi K., Shiokawa H. Crystallization and properties of creatine kinase from equine skeletal muscle. J Biochem. 1981 May;89(5):1619–1631. doi: 10.1093/oxfordjournals.jbchem.a133357. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasák M., Nagayama K., Wüthrich K., Mertens M. L., Kägi J. H. Creatine kinase. Nuclear magnetic resonance and fluorescence evidence for interaction of adenosine 5'-diphosphate with aromatic residue(s). Biochemistry. 1979 Nov 13;18(23):5050–5055. doi: 10.1021/bi00590a004. [DOI] [PubMed] [Google Scholar]