Figure 1.
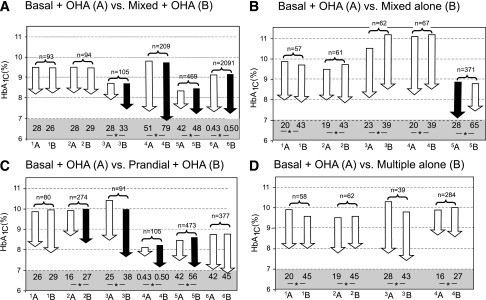
Graphical representation of studies comparing basal insulin plus OHA with premixed insulin plus OHA (A), premixed insulin alone (B), prandial insulin plus OHA (C), and multiple insulin alone (D). Paired comparisons in each panel represent two insulin therapy arms: basal insulin plus OHA (A) and the comparator arm in each pair (B). Arrows indicate baseline and end HbA1c in each study. A black arrow indicates a significant difference in HbA1c compared with the other arm and a white arrow a nonsignificant difference. The numbers below the arrows denote total insulin doses (IU/day). *Significant difference in insulin doses between the two arms. The total number of subjects in the two treatment arms is shown above the arrows. For panel A, the studies are as follows: 1A, Kilo et al. (ref. 20), bedtime NPH × 1 and metformin; 1B, biphasic protaminated aspart/aspart 70/30 × 2 + metformin; 2A, Kilo et al., bedtime NPH × 1 and metformin; 2B, protaminated human insulin/human insulin 70/30 × 2 + metformin (ref. 20); 3A, Malone et al. (ref. 21), bedtime glargine × 1 + metformin; 3B, lispro protamine suspension/lispro 75/25 × 2 + metformin; 4A, Raskin et al. (ref. 22), bedtime glargine × 1 + metformin + PIO (pioglitazone) (∼30%); 4B, biphasic insulin aspart 70/30 × 2 + metformin + PIO (∼30%); 5A, Holman et al. (ref. 17), detemir × 1–2 + metformin + sulfonylurea; 5B, 70/30 aspart × 2 + metformin + sulfonylurea; 6A, Buse et al. (ref. 23), glargine × 1 + OHA; and 6B, protamine suspension 75% and lispro 25% × 2. For panel B, studies are as follows: 1A, Yki-Järvinen et al. (ref. 24), bedtime NPH + metformin + sulfonylurea vs. 1B, NPH/regular 70/30 × 2; 2A, Yki-Järvinen et al. (ref. 24), morning NPH + metformin + sulfonylurea, vs. 2B, NPH/regular 70/30 × 2; 3A, Wolffenbuttel et al. (ref. 25), bedtime NPH × 1 + sulfonylurea; 3B, NPH/regular 70/30 × 2; 4A, Wolffenbuttel et al. (ref. 25), morning NPH × 1 + sulfonylurea, vs. 4B, NPH/regular 70/30 × 2; 5A, Janka et al. (ref. 12), morning glargine × 1 + sulfonylurea + metformin, vs. 5B, NPH/regular 70/30 × 2. For panel C, the studies are as follows: 1A, Landstedt-Hallin et al. (ref. 26), bedtime NPH × 1 + sulfonylurea, vs. 1B, regular × 3 + sulfonylurea; 2A, Bastyr et al. (ref. 27), bedtime NPH + sulfonylurea, vs. 2B, lispro × 3 + sulfonylurea; 3A, Bastyr et al. (ref. 28), bedtime NPH × 1 + sulfonylurea, vs. 3B, lispro × 3 + sulfonylurea; 4A, Kazda et al. (ref. 29), glargine, vs. 4B, lispro × 3; 5A, Holman et al. (ref. 17), detemir × 1–2 + sulfonylurea + metformin, vs. 5B, aspart × 3 + sulfonylurea + metformin; and 6A, Bretzel et al. (ref. 30), glargine × 1 + sulfonylurea + metformin, vs. 6B, lispro × 3 + sulfonylurea + metformin. For panel D, the studies are as follows: 1A, Yki-Järvinen et al. (ref. 24), bedtime NPH + sulfonylurea + metformin, vs. 1B, regular × 3 and NPH; 2A, morning NPH + sulfonylurea + metformin, vs. 2B, regular × 3 and NPH (ref. 24); 3A, Clauson et al. (ref. 31), bedtime NPH and sulfonylurea, vs. 3B, rapid-acting insulin × 3 and NPH; and 4A, Bastyr et al. (ref. 27), bedtime NPH + sulfonylurea, vs. 4B, lispro × 3 + NPH.
