Abstract
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) acts as an apoptosis inducer for cancer cells sparing non-tumor cell targets. However, several phase I/II clinical trials have shown limited benefits of this molecule. In the present work, we investigated whether cell susceptibility to TRAIL ligation could be due to the presence of TRAIL death receptors (DRs) 4 and 5 in membrane microdomains called lipid rafts. We performed a series of analyses, either by biochemical methods or fluorescence resonance energy transfer (FRET) technique, on normal cells (i.e. lymphocytes, fibroblasts, endothelial cells), on a panel of human cancer B-cell lines as well as on CD19+ lymphocytes from patients with B-chronic lymphocytic leukemia, treated with different TRAIL ligands, that is, recombinant soluble TRAIL, specific agonistic antibodies to DR4 and DR5, or CD34+ TRAIL-armed cells. Irrespective to the expression levels of DRs, a molecular interaction between ganglioside GM3, abundant in lymphoid cells, and DR4 was detected. This association was negligible in all non-transformed cells and was strictly related to TRAIL susceptibility of cancer cells. Interestingly, lipid raft disruptor methyl-beta-cyclodextrin abrogated this susceptibility, whereas the chemotherapic drug perifosine, which induced the recruitment of TRAIL into lipid microdomains, improved TRAIL-induced apoptosis. Accordingly, in ex vivo samples from patients with B-chronic lymphocytic leukemia, the constitutive embedding of DR4 in lipid microdomains was associated per se with cell death susceptibility, whereas its exclusion was associated with TRAIL resistance. These results provide a key mechanism for TRAIL sensitivity in B-cell malignances: the association, within lipid microdomains, of DR4 but not DR5, with a specific ganglioside, that is the monosialoganglioside GM3. On these bases we suggest that lipid microdomains could exert a catalytic role for DR4-mediated cell death and that an ex vivo quantitative FRET analysis could be predictive of cancer cell sensitivity to TRAIL.
Keywords: cancer, hematology, TRAIL, death receptors, lipid rafts, apoptosis
In the course of years, several attempts have been made in order to develop therapeutic strategies aimed at the induction of cell death program, that is, apoptosis. Unfortunately, clinical benefits deriving from this approach appeared as limited. Instead, more recently, the use of biological agents as well as of the so-called molecularly targeted therapy lead to some promising clinical result. In particular, agents capable of specifically modulating cell signals leading to apoptosis of cancer cells sparing non-tumor cell targets gained the attention of physicians and several studies have been carried out by both experimental and clinical points of view. Among these are works dealing with chemical or biological agents capable of activating tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). TRAIL is a member of the tumor necrosis factor (TNF) superfamily, highly homologous to CD95/Fas, which is expressed by several cell types. TRAIL ligates two types of receptors: death receptor (DRs), triggering apoptosis, and decoy receptors, which negatively modulate this pathway.1, 2 To date, four human receptors specific for TRAIL have been identified: the DRs TRAILR1 (DR4) and TRAILR2 (DR5), and the putative decoy receptors TRAILR3 (DCR1) and TRAILR4 (DCR2).3
Binding of TRAIL, or administration of agonistic antibodies to DR4 or DR5, resulted in receptor oligomerization and initiation of apoptosis via recruitment of membrane-proximal caspases (caspase 8 or caspase 10).4 Furthermore, it has previously been suggested that death receptors can be recruited into specific plasma membrane regions called lipid rafts that could facilitate protein–protein interactions and convey apoptotic signals.5, 6 Lipid rafts are plasma membrane microdomains varying in size from 50 to 70 nm, enriched in cholesterol and sphingolipids, and having many important roles in cell signal transduction. In fact, proteins located in these microdomains are severely limited in their ability to freely diffuse over the plasma membrane,7 and their concentration and association into lipid rafts can influence their function and signaling.8, 9 It has been hypothesized that cell treatment with TRAIL could trigger the redistribution of receptors DR4 and/or DR5 into lipid rafts potentiating apoptosis.10, 11, 12
Promising preclinical results using either recombinant soluble TRAIL or agonistic death receptor antibodies prompted phase I/II clinical trials exploring the safety and efficacy of these treatments. However, although these clinical studies have demonstrated a good toxicity profile, the major caveat was the highly variable response of cancer cells to apoptosis induction.
In the present study, we investigated in several human cell types, including non-transformed cells, as well as in CD19+ lymphocytes from patients with B-chronic lymphocytic leukemia, the possible implication of lipid raft microdomains in determining cell susceptibility to TRAIL-induced apoptosis. We show that the constitutive localization of TRAIL receptor DR4 into lipid rafts could represent a critical variable causing anticancer activity of TRAIL. The mechanism for this sensitivity seems to be based on the association, within lipid microdomains, of DR4, but not DR5, with a specific ganglioside, that is, the monosialoganglioside monoganglioside 3 (GM3). This molecular interaction appears as a prerequisite for cell susceptibility to TRAIL-induced apoptosis.
Results
Surface expression of TRAIL receptors in transformed and non-transformed cells
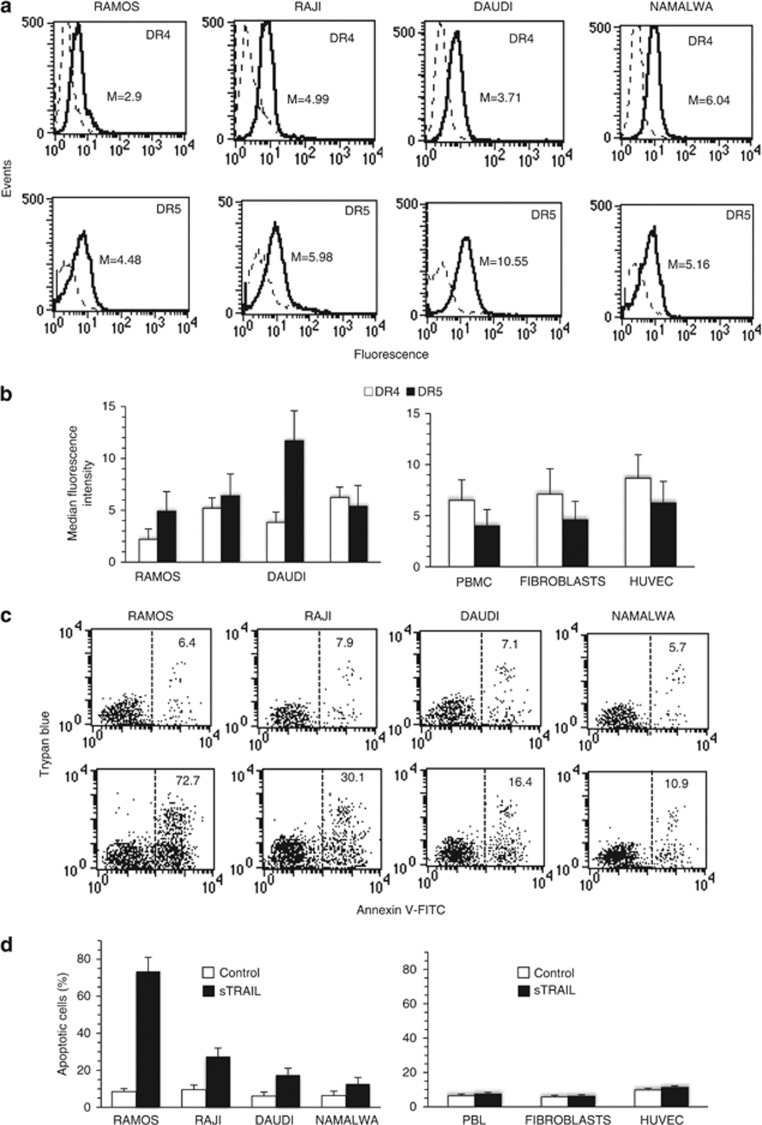
As reported in Figure 1a and Figure 1b, surface expression levels of DR4 and DR5 receptors in different Burkitt's lymphoma cell lines (Ramos, Raji, Daudi, Namalwa) were variable. In the same vein, in non-transformed cells, such as freshly isolated human peripheral blood lymphocytes (PBL), human fibroblasts and human umbilical vein endothelial cells (HUVEC), surface expression of DR4 and DR5 was variable, although DR4 appeared significantly more expressed than DR5 (Figure 1b, right panel).
Figure 1.
(a) Surface expression of TRAIL receptors in transformed and non-transformed cells. Flow cytometry analysis of surface expression level of DR4 and DR5 in four Burkitt lymphoma cell lines. Histograms obtained in a representative experiment are shown. Numbers represent the median fluorescence intensity. Results obtained in a representative experiment are shown. (b) Graphs showing the surface expression level of DR4 and DR5 in four Burkitt lymphoma cell lines (left panel) and in non-transformed cells PBL, fibroblasts and HUVEC (right panel). Mean±S.D. of the median fluorescence intensity obtained in four different experiments is reported. (c) Soluble TRAIL-induced apoptosis in transformed and non-transformed cells. Biparametric flow cytometry analysis of sTRAIL-induced apoptosis in four Burkitt lymphoma cell lines after double staining with annexin V-FITC/Trypan blue. Numbers represent the percentage of dead cells either annexin V/Trypan blue double-positive or annexin V single-positive. Results obtained in a representative experiment are shown. (d) Bar graphs showing the amount of apoptosis in four Burkitt lymphoma cell lines (left panel) and in non-transformed PBL, fibroblasts and HUVEC (right panel) after sTRAIL administration. Mean±S.D. of the percentages of annexin V-positive cells obtained in four different experiments is reported. Significant differences (P<0.01) were detected between control and treated cancer cells
Soluble TRAIL-induced apoptosis in transformed and non-transformed cells
Quantitative flow cytometry analysis revealed a different susceptibility in terms of sTRAIL-induced apoptosis among various lymphoma cell lines, that is, Ramos cells being highly susceptible, Daudi and Namalwa cells resistant, whereas Raji cells showed an intermediate sensitivity to TRAIL (Figures 1c and d, left panel). As far as non-transformed cells were concerned, PBL, fibroblasts and HUVEC did not show any sign of apoptosis following sTRAIL administration (Figures 1c and d, right panel). Thus, TRAIL-induced apoptotic susceptibility and resistance appeared to be unrelated to DR4 and DR5 surface expression levels.
We also analyzed two further tumor cell lines: CEM (T-cell leukemia) and HD-MyZ (histiocytic cell line). We observed a different susceptibility of these cell lines to sTRAIL (Supplementary Results and Supplementary Figure 1B). In particular, CEM cells were scarcely susceptible to this cytokine (<15% of apoptotic cells), whereas HD-MyZ appeared more susceptible to sTRAIL (about 25% of apoptotic cells). No correlation was however detectable between DR4 and DR5 surface expression levels (Supplementary Results and Supplementary Figure 1A) and TRAIL susceptibility.
Constitutive localization of TRAIL receptors into lipid rafts
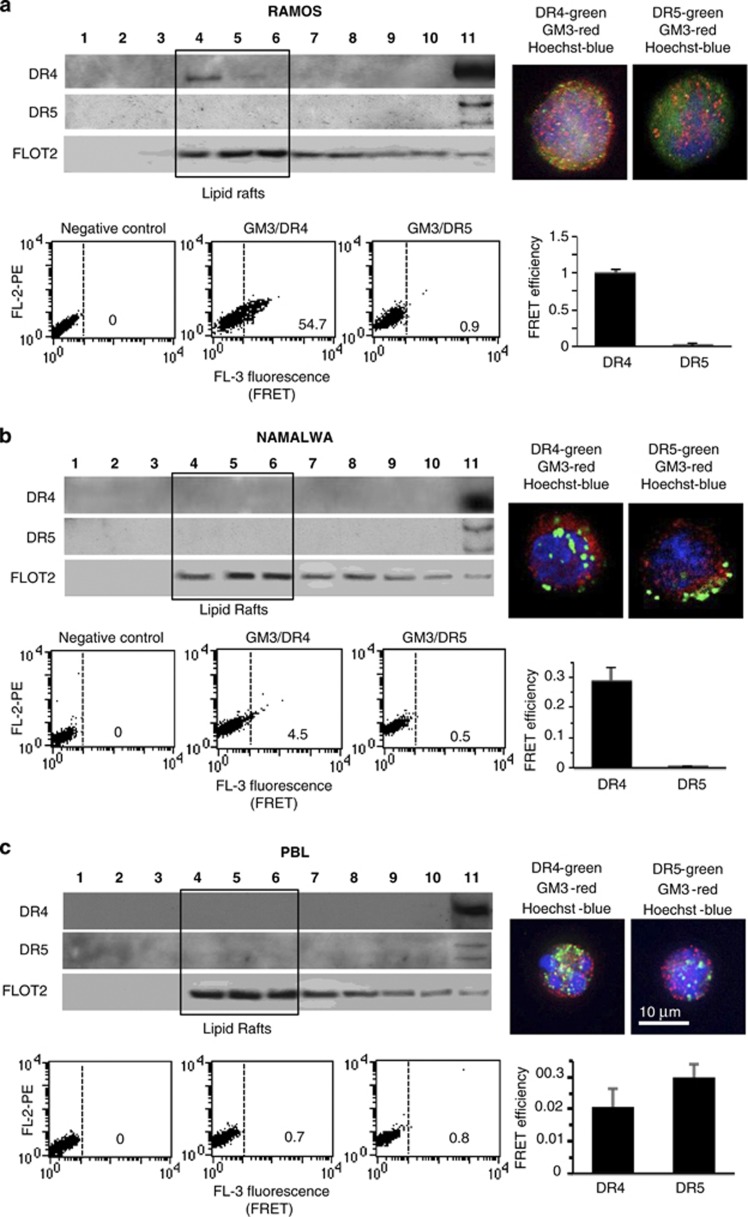
Lipid rafts serve to concentrate specific receptors at the cell plasma membrane also providing a scaffold for signal transduction proteins. For instance, it has been reported that the recruitment into lipid rafts of the major DRs, including DR4 and DR5, together with their downstream signaling molecules, for example, caspase 8 initiator-caspase, can promote apoptosis.13, 14 On these bases, we analyzed the distribution of DR4 and DR5 in fractions obtained by a 5–30% discontinuous sucrose gradient in the two Burkitt's lymphoma cell lines showing dramatically different susceptibilities to TRAIL: Ramos (highly susceptible) and Namalwa (resistant), and in non-transformed cells, that is, PBL (resistant to TRAIL). As shown in Figure 2a (left box), DR4 was constitutively present in fraction 4 in Ramos cells (corresponding to raft microdomains) as well as in fractions 10 and 11 (corresponding to soluble fractions). By contrast, DR5 was detectable only in fractions 10 and 11 but was virtually absent in the fractions 4–6 corresponding to raft microdomains (marked by flotillin 2, (FLOT2)). By contrast, in Namalwa cells (Figure 2b, left panel), as well as in PBL (Figure 2c, left panel), either DR4 or DR5 appeared almost completely restricted at detergent-soluble fractions 10 and 11.
Figure 2.
Constitutive localization of TRAIL receptors into lipid rafts. Left panels. Western blot analysis of sucrose gradient fractions. Fractions obtained after sucrose density gradient were analyzed by using anti-DR4 antibodies (first line) and anti-DR5 antibodies (second line). The third lane shows flotillin 2 (FLOT2) distribution, known to be enriched in fractions corresponding to lipid rafts (fractions 4–6). Right panels. IVM analysis after triple cell staining with anti-DR4/anti-GM3/Hoechst (upper panel) or with anti-DR5/anti-GM3/Hoechst. The yellow fluorescence areas indicate the co-localization. Bottom boxes. Quantitative evaluation of GM3/DR4 and GM3/DR5 association by FRET technique, as revealed by flow cytometry analysis. Numbers represent the FRET efficiency (calculated by using Rieman algorithm) and indicate that GM3/DR4 association was higher in Ramos cells, very low in Namalwa cells and negligible in PBL. GM3/DR5 association was absent in Ramos, Namalwa and PBL. Results obtained in one experiment representative of three are shown. (a) Ramos lymphoma cell line, (b) Namalwa lymphoma cell line and (c) freshly isolated human lymphocytes (PBL). Note different scales in FE bar graphs
The localization of DR4 into lipid rafts in Ramos cells was further confirmed by immunofluorescence analysis performed by intensified video microscopy (IVM) that showed an evident co-localization of DR4 with GM3, a glycosphingolipid that is abundant in lipid rafts of these cells (Figure 2a, right panel, yellow fluorescence) that we considered as a paradigmatic ganglioside in this study. According to biochemical data, in Namalwa cells and in PBL, IVM analysis did not reveal any co-localization of GM3 neither with DR4 nor with DR5 (Figures 2b and c, right panels).
These results, obtained by using biochemical methods, were also confirmed by using the FRET technique (bottom boxes). The sensitivity of this approach allowed us to investigate the molecular association of GM3 with DR4 or DR5 in entire non-permeabilized cells.15 This quantitative analysis indicated a strict molecular interaction of GM3 with DR4 in susceptible cells only, for example, Ramos cells (Figure 2a, bottom box), as also demonstrated by FRET efficiency (FE) calculation performed by pooling together results obtained from three independent experiments (Figure 2a, bottom box, bar graph). Interestingly, also in Namalwa cells, we observed some association between GM3 and DR4, although significantly lower in comparison to Ramos cells (Figure 2b, bottom box). By contrast, in freshly isolated PBL, the molecular interaction between GM3 and DR4 was completely absent (Figure 2c, bottom box). According to biochemical data, neither in Ramos nor in Namalwa cells and PBL we found any significant association of GM3 with DR5.
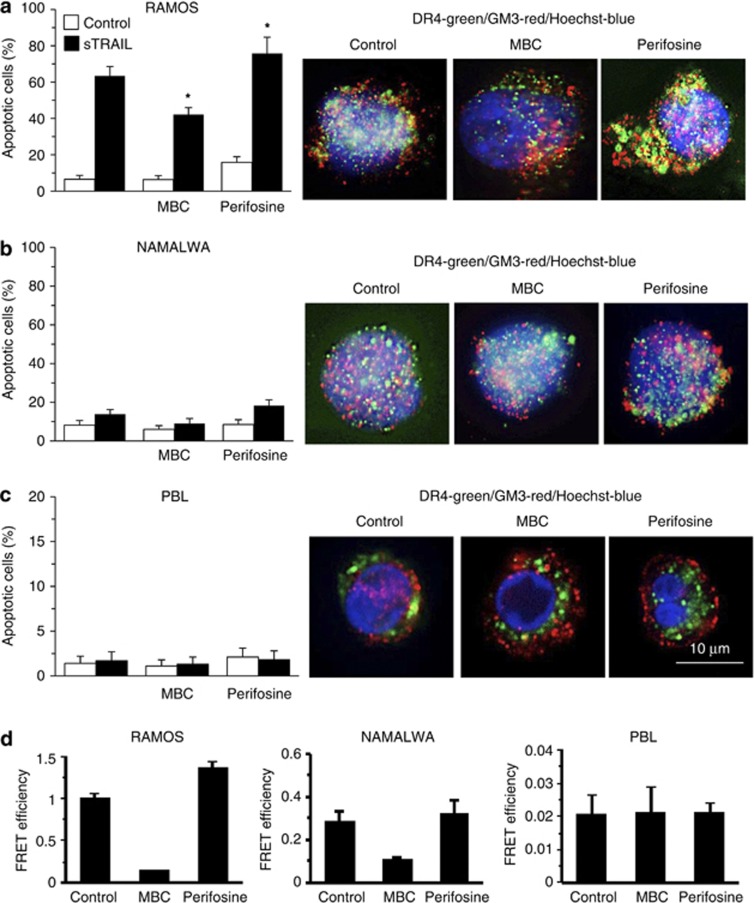
Lipid rafts and TRAIL-induced apoptosis
On the basis of the results reported above, two different agents capable of perturbating microdomain integrity have been considered: methyl-beta-cyclodextrin (MBC), which disrupts lipid rafts by removing cholesterol from membranes16 and the anticancer agent perifosine (a synthetic alkyl-lysophospholipid), which is known to favor the recruitment of DRs into the lipid rafts.3, 17 We found that MBC, when administered before sTRAIL, was capable of significantly hindering apoptosis in Burkitt's lymphoma cell line Ramos (Figure 3a, left panel) and was ineffective in Namalwa cells (Figure 3b, left panel) as well as in PBL (Figure 3c, left panel). By contrast, perifosine was able to significantly (P<0.05) increase sTRAIL-induced apoptosis in Ramos cell line, and, although to a lesser extent, also in the refractory cell line Namalwa (Figure 3b) but failed to induce any significant effect in PBL (Figure 3c) and in other non-tumor cells (i.e. fibroblasts and HUVEC, not shown). Importantly, neither MBC nor perifosine were able to significantly modify the cell surface expression levels of DR4 and DR5 in all cell types mentioned above (not shown).
Figure 3.
Lipid rafts and TRAIL-induced apoptosis. Left panels. Bar graphs showing the amount of sTRAIL-induced apoptosis in cells pretreated with MBC or perifosine. Mean±S.D. of the percentages of annexin V-positive cells obtained by flow cytometry in four different experiments is reported. Right panels. IVM analysis after triple cell staining with anti-DR4/anti-GM3/Hoechst in control, MBC- or perifosine-treated cells. The yellow fluorescence areas indicate the co-localization. (a) Ramos lymphoma cell line, (b) Namalwa lymphoma cell line and (c) freshly isolated human lymphocytes. * indicates P<0.01 ° indicates P<0.05 versus sTRAIL samples. (d) Quantitative evaluation of GM3/DR4 and GM3/DR5 association by FRET technique, as revealed by flow cytometry analysis. Numbers represent the FRET efficiency (calculated by using Riemann algorithm). Note different scales
According to these data, IVM analysis showed that co-localization of DR4 with ganglioside GM3 observed in control Ramos cells (Figure 3a, left micrograph) was completely lost after treatment with MBC (Figure 3a, central micrograph) and it was emphasized by perifosine treatment (Figure 3a, right micrograph). In Namalwa cell line, IVM analysis did not reveal any co-localization of GM3 with DR4 either in control (Figure 3b, left micrograph) or in MBC-treated cells (Figure 3b, central micrograph) but after treatment with perifosine (Figure 3b, right micrograph), a partial co-localization of GM3 and DR4, which was paralleled by an increased sTRAIL-induced apoptotic response (Figure 3b, left panel) was observed. However, according to apoptosis data, perifosine was anyway significantly more effective in Ramos cells than in Namalwa cells. No co-localization at all was detectable in PBL (Figure 3c, right micrograph). Quantitative analysis performed by the FRET technique by application of Riemann's algorithm to evaluate FE (Figure 3d) indicated that the strict molecular interaction of GM3 with DR4 observed in Ramos cells was emphasized by perifosine treatment and significantly impaired by MBC administration (Figure 3d, left panel). In Namalwa cells, in which we observed a minimal association between GM3 and DR4, we found a small, statistically non-significant increase of this molecular association after perifosine treatment. A substantial decrease of GM3/DR4 association was also observed after MBC administration (Figure 3d, central panel). By contrast, in freshly isolated PBL these two drugs did not influence the GM3/DR4 interaction significantly (Figure 3c, right panel).
Hence, raft disruptor MBC modified apoptotic susceptibility only in cells in which DRs are already in microdomains, whereas the raft-recruiting agent perifosine increases TRAIL susceptibility only in those cells that are able to recruit DR4 into lipid rafts. HUVEC and fibroblasts, which did not display any constitutive molecular association of GM3 with DR4 or DR5, were also refractory to perifosine ‘booster' activity (not shown). An exemplification of FE computation by Riemann's algorithm is reported in Supplementary Files 1 and 2.
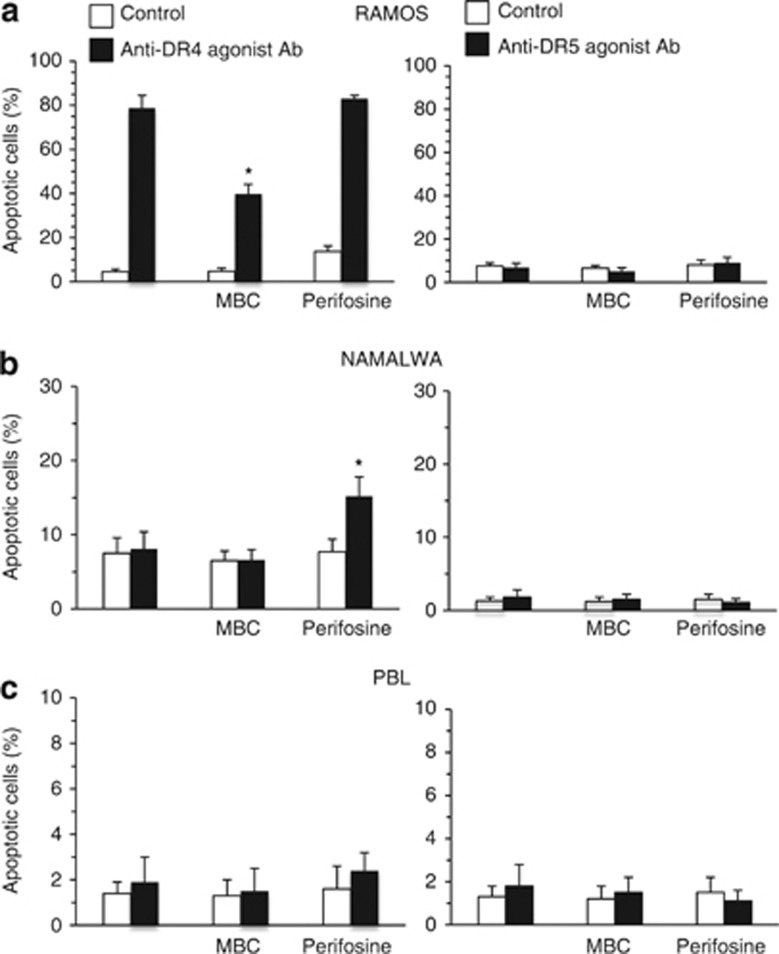
Apoptotic induction by DR4 and DR5 agonist antibodies
Besides, we evaluated pro-apoptotic effects of agonist antibodies to DR4 and DR5 in Ramos and Namalwa lymphoma cell lines as well as in PBL (Figure 4). As expected on the basis of the above results, we found that only DR4 agonist antibodies induced apoptosis in Ramos cell line, whereas agonist antibodies to DR5 were ineffective (Figure 4a). By contrast, no significant apoptosis was observed in Namalwa cell line (Figure 4b) and in PBL (Figure 4c) either treated with anti-DR4 or anti-DR5 agonist antibodies. According to the results obtained by using sTRAIL, we also observed that MBC significantly hindered apoptosis induced by DR4 agonist antibodies, whereas perifosine favored DR4 agonist antibodies-induced cell death. By contrast, perifosine did not influence anti-DR5-induced apoptosis either in Ramos or in Namalwa cell lines. Importantly, treatments of PBL either with MBC or with perifosine did not induce any significant change in apoptosis values.
Figure 4.
Apoptotic induction by DR4 and DR5 agonist antibodies. Bar graphs showing the amount of apoptosis induced by anti-DR4 (left panels) and anti-DR5 (right panels) agonist antibodies in control cells and in cells pretreated with MBC or perifosine before apoptotic triggering. Mean±S.D. of the percentages of annexin V-positive cells obtained by flow cytometry in four different experiments is reported. (a) Ramos lymphoma cell line; (b) Namalwa lymphoma cell line and (c) freshly isolated human lymphocytes. * indicates P<0.01 versus anti-DR4 agonist Ab samples
CD34-TRAIL+ induced apoptosis in transformed and non-transformed cells
It has been reported that membrane-bound TRAIL (mTRAIL) is more active than sTRAIL in inducing apoptosis.18 In addition, it was recently published by our group that adenovirus-transduced CD34+ cells overexpressing mTRAIL (CD34-TRAIL+ cells) exerted a potent antitumor activity in vivo.19 We therefore tested the ability of these armed cells (CD34+-armed cells) to induce cell death in our panel of cell types, that is, either in cancer or, more importantly, in non-cancer cells. The following human cells were used as target in these experiments: Burkitt's lymphoma cell lines Ramos, Raji, Daudi and Namalwa, the T-cell leukemia CEM and HD-MyZ histiocytic cell line. As non-transformed cells we tested PBL and HUVEC (Supplementary Results and Supplementary Figure 2). Coculturing CD34-TRAIL cells with target cells mentioned above for 48 h (1 : 1 effector:target cell ratio) resulted in a highly significant induction of apoptosis. In particular, we observed that although the various target cells showed a different susceptibility to sTRAIL, treatment with mTRAIL, that is, with CD34+-TRAIL-armed cells, was significantly more effective in inducing cell death than sTRAIL (at least at the dose of 200 ng/ml as stated in the literature).20 Importantly, TRAIL-armed CD34+ cells were not able to induce cell death in non-transformed cells, that is, PBL and HUVEC (Supplementary Results and Supplementary Figure 2).
Ex vivo analyses of lymphocytes isolated from patients with chronic lymphoblastic leukemia
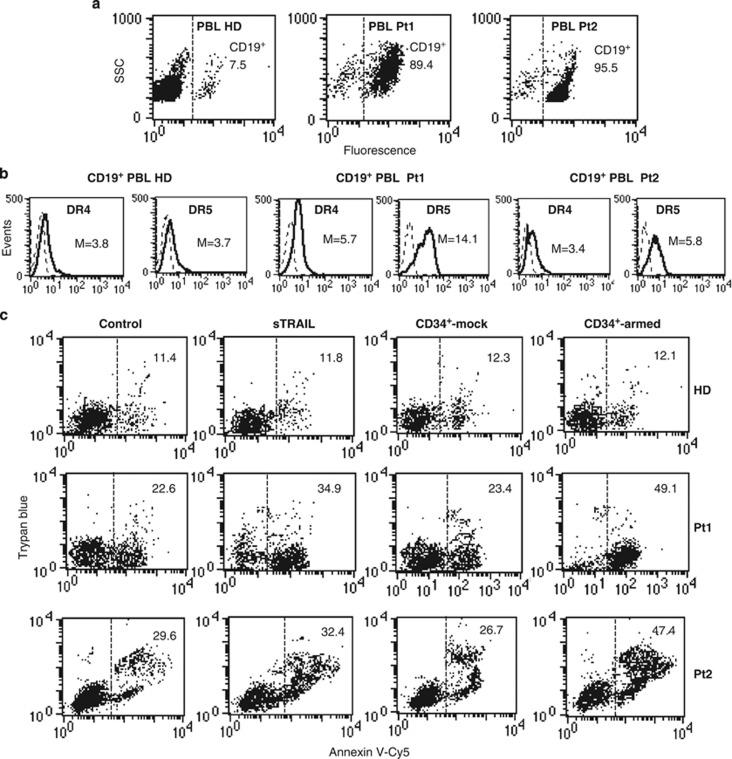
In order to verify the forcefulness of our hypothesis, that is, whether the constitutive association of TRAIL receptor with microdomains could be predictive of the response to therapy, an ex vivo investigation has been carried out. We analyzed lymphocytes isolated from the peripheral blood of six untreated chronic lymphoblastic leukemia (CLL) patients (Pt1-Pt6). We compared lymphocytes isolated from these patients with those isolated from healthy donors (HD) in terms of: (i) surface expression of TRAIL receptors; (ii) susceptibility to sTRAIL- and mTRAIL-induced apoptosis; (iii) susceptibility to apoptotic induction by DR4 and DR5 agonist antibodies; (iv) localization of TRAIL receptors into lipid rafts (by FRET analysis) and (v) the possibility of modulating TRAIL-induced apoptosis of ex vivo collected cells by modulating lipid rafts. All our analyses were restricted to B-cell population as pinpointed by using anti-CD19 antibodies. In fact, we found that the percentage of CD19-positive cells in healthy donors varied from about 7 to 12%, as expected (Figure 5a, first panel shows results obtained in a representative donor), whereas in PBL derived from pathological subjects the percentage of CD19-positive cells was of more than 75% (Figure 5a).
Figure 5.
(a) Ex vivo analyses of lymphocytes isolated from patients with CLL. Flow cytometry analysis of surface expression level of CD19 in lymphocytes freshly isolated from a representative HD among six or from two pathological subjects among six (Pt1 and Pt2) affected by B leukemia. Numbers represent the percentage of CD19-positive cells. Results obtained in a representative experiment are shown. (b) Surface expression of TRAIL receptors. Flow cytometry analysis of surface expression level of DR4 and DR5 in lymphocytes freshly isolated from a healthy donor or from two pathological subjects affected by B leukemia. Numbers represent the median fluorescence intensity. Results obtained in a representative experiment are shown. (c) Apoptosis induction by sTRAIL, mTRAIL and agonist antibodies to DR4 and DR5. Biparametric flow cytometry analysis of apoptosis induced by sTRAIL or by mTRAIL, that is, coculturing CD34+-armed cells or CD34+-mock with target cells (1 : 1 ratio). Numbers represent the percentage of apoptotic cells either annexin V/Trypan blue double-positive or annexin V single-positive. Results obtained in a representative experiment are shown. Analyses shown in b and c were restricted to CD19-positive cells
Surface expression of TRAIL receptors
Analysis of CD19-positive living lymphocytes showed that surface expression levels of both TRAIL DRs DR4 and DR5 were higher in B lymphocytes isolated from pathological subjects than in B cells derived from healthy donors. In addition, we also observed that DR5 expression level in B lymphocytes of these pathological subjects was significantly higher than DR4 (Figure 5b).
Apoptosis induction by sTRAIL, mTRAIL and agonist antibodies to DR4 and DR5
When apoptotic susceptibility to sTRAIL and mTRAIL was evaluated (Figure 5c), we found that B lymphocytes isolated from healthy donors were resistant either to sTRAIL or mTRAIL (first row). As far as pathological subjects were concerned, we found that B lymphocytes isolated from four of these were quite susceptible to TRAIL-induced apoptosis (results from a representative patient are shown in Figure 5), whereas B lymphocytes derived from the other two patients were almost completely resistant to TRAIL (results from a representative patient are shown in Figure 5). As reported above in B lymphoma cell lines, also in lymphocytes freshly isolated from peripheral blood, mTRAIL (i.e. CD34+-armed cells) was more effective than sTRAIL in inducing cell death (compare Supplementary Figure 2 with Figure 6c).
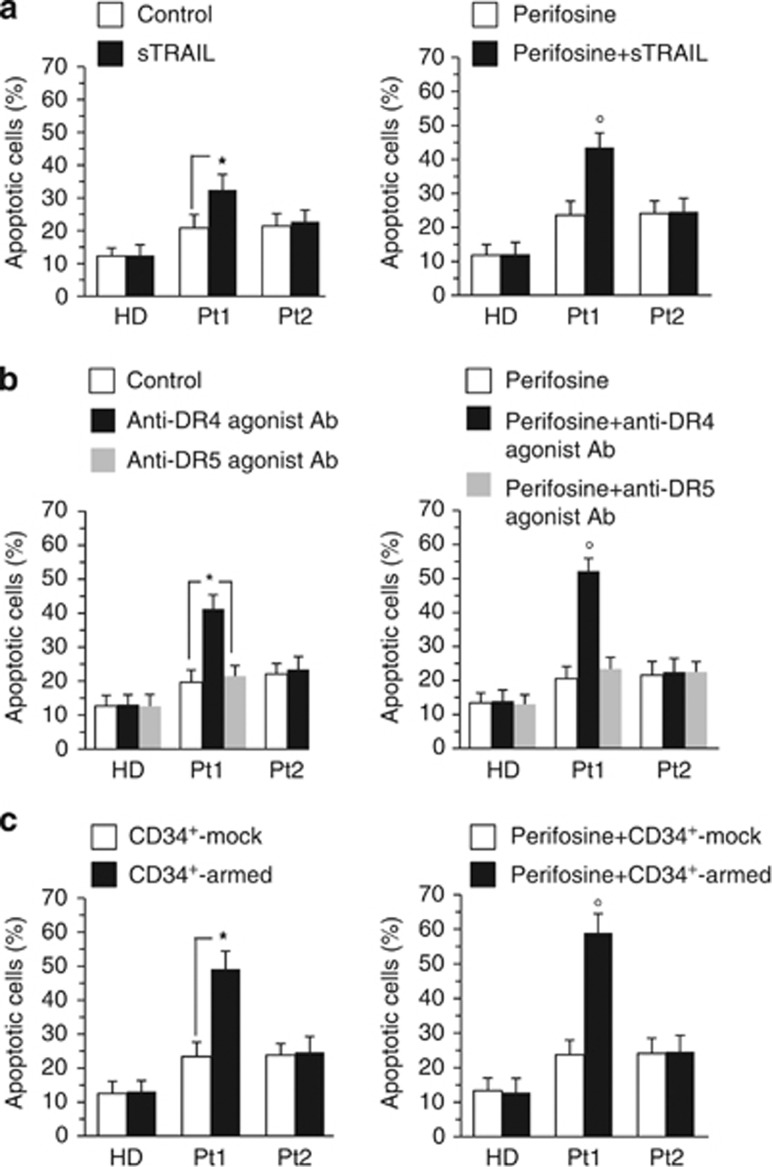
Figure 6.
Ex vivo analyses of lymphocytes isolated from patients with CLL. Flow cytometry analysis of lymphocytes freshly isolated from a representative HD among six or from two pathological subjects among six affected by B leukemia (Pt1 and Pt2) triggered for apoptosis. Bar graphs showing the amount of apoptosis induced by (a) sTRAIL, (b) anti-DR4 or anti-DR5 agonist antibodies or (c) coculturing CD34+-armed cells with target cells (1 : 1 ratio) in control cells and in cells pretreated with perifosine before apoptotic triggering. Note that representative Pt1 was susceptible to all the pro-apoptotic stimuli mentioned above. Mean±S.D. of the percentages of annexin V-positive cells obtained in four different experiments is reported. * indicates P<0.01; ° indicates P<0.05 versus sTRAIL sample (a), versus anti-DR4 agonist Ab sample (b) or versus CD34+-armed sample (c)
Further analyses also demonstrated that: (i) DR4 but not DR5 agonist antibodies were able to induce apoptosis in B lymphocytes derived from susceptible patients (representative results, patient 1, Pt1, are shown in Figure 6b, left panel) and that (ii) perifosine induced a significant increase (P<0.05) of apoptosis ignited by sTRAIL, mTRAIL and DR4 agonist antibodies, although at higher concentrations with respect to B lymphoma-cultured cell lines (Figures 6a–c, respectively, right panels).
Localization of TRAIL receptors into lipid rafts
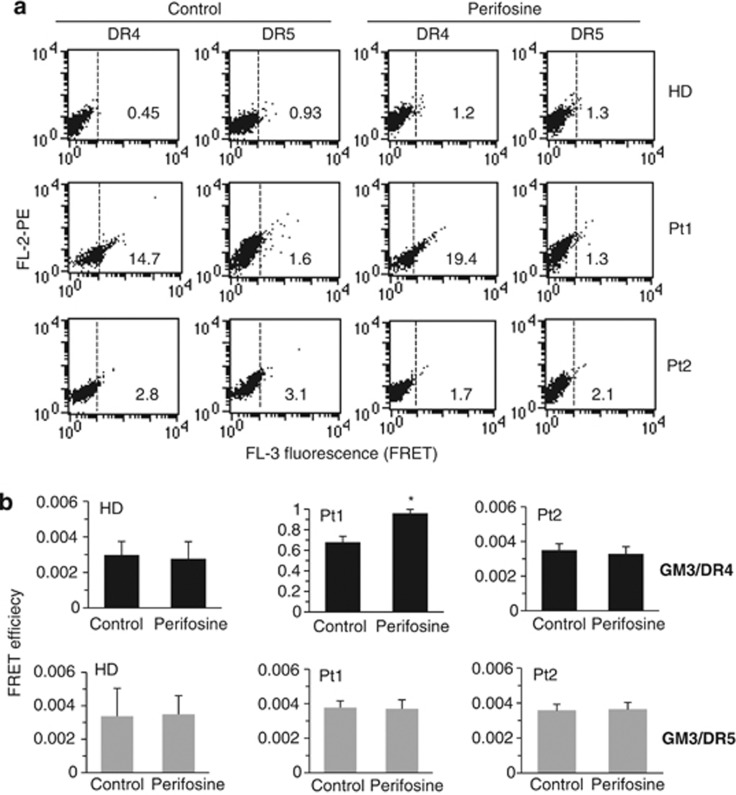
We investigated the molecular association of GM3 with DR4 or DR5 by quantitative FRET analysis. These analyses were conducted on a cell population stained for: CD19 (FITC, FL1), DR4 or DR5 (PE, FL2) and GM3 (Cy5, FL4). To note, FRET was evaluated in FL3 fluorescence channel restricting our analysis to CD19-positive cells only.
As reported in Figure 7, according to apoptosis data, we found a constitutive association of DR4 (but not of DR5) with GM3 in B lymphocytes susceptible to TRAIL-induced apoptosis (representative plots in Figure 7a). By contrast, this association was negligible either in B lymphocytes isolated from healthy donors (one representative of six is shown) or from patients who were resistant to TRAIL (representative plots in Figure 7a). As far as perifosine was concerned, we found an increase of GM3/DR4 association in cells treated with this drug with respect to controls (P<0.05). Quantitative analysis of FRET data by application of Riemann's algorithm to evaluate FE from three independent experiments (Figure 7b) also indicated that the molecular interaction of GM3 with DR4 (black columns) observed in B lymphocytes isolated from patients susceptible to TRAIL was significantly increased by treatment with perifosine (P<0.05). The association of GM3 with DR5 (gray columns) was negligible either in B lymphocytes isolated from healthy donors or from pathological subjects. In Table 1 are summarized results obtained in four Burkitt's lymphoma cell lines (Ramos, Raji, Daudi and Namalwa) in comparison with non-transformed cells (i.e. PBL, fibroblasts and HUVEC) and with B lymphocytes isolated from six HD and from six untreated B-CLL patients (Pt1-Pt6).
Figure 7.
Localization of TRAIL receptors into lipid rafts. (a) Flow cytometry analysis of GM3/DR4 or GM3/DR5 association by FRET technique in lymphocytes freshly isolated from a representative HD among six or from two pathological subjects among six affected by B leukemia (Pt1, susceptible, and, Pt2, resistant). Analyses were performed in control cells and in cells treated with perifosine. Numbers indicate the percentage of FL3-positive events obtained in one experiment representative of three. (b) Bar graphs showing evaluation of FE, according to the Riemann's algorithm, of GM3/DR4 and GM3/DR5 association. Results shown represent the mean±S.D. from three independent experiments. Statistical analyses indicated that: (i) GM3/DR4 association was significant in lymphocytes isolated from Pt1 (susceptible to pro-apoptotic stimuli, see Figure 6) but not in lymphocytes from HD and Pt2 (resistant to pro-apoptotic stimuli, see Figure 6) (black columns); and that (ii) GM3/DR5 association was irrelevant either in lymphocytes from HD or from pathological subjects (gray columns). Note different scales. * indicates P<0.05 versus control samples
Table 1. Quantitative flow cytometry analyses of cell death and TRAIL receptors/GM3 association: in vitro and ex vivo experiments.
|
Cell death |
FRET efficiency |
|||||
|---|---|---|---|---|---|---|
|
Tumor cells | ||||||
| sTRAIL | CD34-armed | α-DR4 | α-DR5 | DR4/GM3 | DR5/GM3 ( × 10−3) | |
| Ramos | 64.9±5.3 | 72.5±6.7 | 74.3±8.3 | 1.2±0.4 | 1.00±0.05 | 2.7±2.2 |
| Raji | 20.2±4.0 | 32.3±3.6 | 26.7±3.4 | 1.5±0.3 | 0.47±0.04 | 3.3±3.1 |
| Daudi | 11.4±3.7 | 22.9±3.1 | 16.8±2.7 | 1.0±0.5 | 0.49±0.03 | 4.1±4.1 |
| Namalwa | 7.1±3.1 | 12.2±2.7 | 5.9±2.1 | 1.3±0.2 | 0.29±0.04 | 4.3±1.3 |
|
Non-transformed cells | ||||||
|---|---|---|---|---|---|---|
| sTRAIL | CD34-armed | α-DR4 | α-DR5 | DR4/GM3 ( × 10–4) | DR5/GM3 ( × 10–4) | |
| PBL | 1.3±0.7 | 1.5±0.8 | 1.3±0.5 | 1.2±0.8 | 20.1±5.0 | 29.1±4.0 |
| Fibroblasts | 1.1±0.9 | 1.5±0.6 | 1.1±0.7 | 1.0±0.7 | 17.3±2.1 | 19.4±2.2 |
| HUVEC | 1.2±0.7 | 1.7±0.7 | 0.9±0.5 | 1.1±0.5 | 16.2±3.3 | 14.3±3.1 |
|
Ex vivo | ||||||
|---|---|---|---|---|---|---|
| sTRAIL | CD34-armed | α-DR4 | α-DR5 | DR4/GM3 ( × 10–3) | DR5/GM3 ( × 10–4) | |
| HD (n=6) | 0.5±0.2 | 0.6±0.2 | 0.4±0.1 | 0.3±0.1 | 1.7±0.2 | 24.2±4.1 |
| Pt 1 | 22.1±3.4 | 25.7±2.9 | 21.6±2.4 | 0.6±0.2 | 671.5±23.2 | 21.3±2.4 |
| Pt 2 | 1.7±0.5 | 1.5±0.4 | 1.3±0.4 | 0.5±0.2 | 1.9±0.4 | 18.1±1.2 |
| Pt 3 | 19.4±3.4 | 27.5±3.7 | 20.1±2.8 | 1.0±0.3 | 841.6±31.1 | 24.0±2.1 |
| Pt 4 | 18.7±2.5 | 23.1±3.3 | 21.3±2.8 | 1.3±0.8 | 749.1±29.3 | 28.2±1.3 |
| Pt 5 | 2.3±0.9 | 2.1±0.7 | 1.1±0.6 | 1.2±0.6 | 1.7±0.3 | 19.0±2.5 |
| Pt 6 | 21.6±3.5 | 27.9±4.1 | 25.7±3.1 | 1.5±0.7 | 796.4±29.3 | 30.5±3.3 |
Abbreviations: FRET, fluorescence resonance energy transfer; HD, healthy donor; HUVEC, human umbilical endothelial cell; PBL, peripheral blood lymphocytes; TRAIL, Tumor necrosis factor-related apoptosis-inducing ligand
Data from each single patient are reported
Left column: cell death evaluation after apoptosis triggering by sTRAIL, mTRAIL (CD34-armed cells) and by agonist antibodies to DR4 or DR5. Numbers represent the percentage of annexin V-positive cells after subtraction of the percentage of dead cells found in the pertinent control. Right column: quantitative analysis of the molecular association of DR4 or DR5 with GM3 obtained by calculating FRET efficiency by using Riemann's algorithm. Data are reported as mean±S.D. of the results obtained from three independent experiments
Discussion
In the present work, we provide evidence that the constitutive localization of TRAIL DR4 into lipid rafts, that is, in cholesterol-rich triton-insoluble microdomains, could represent per se a prerequisite for a high susceptibility to this cytokine. At variance, its exclusion from microdomains was found as associated to TRAIL resistance, at least in B lymphoblastoid human cells. In fact, we found that: (i) only cells with raft-associated TRAIL receptor DR4 were highly susceptible (e.g. Ramos cells); (ii) the disruption of microdomains (by using MBC) decreased cell susceptibility, whereas (iii) the increased localization of DR4 into lipid rafts (by using perifosine) caused an increased TRAIL-induced cell death. Importantly, according to this, the presence of these receptors into rafts was negligible in normal non-transformed cells (PBL, fibroblasts, endothelial cells, which were resistant to TRAIL) as well as in those cancer cells, which were refractory to TRAIL-induced effects (e.g. lymphoblastoma cell line Namalwa). The specificity of our experimental set up was further reinforced by the use of functional triggers alternative to sTRAIL molecule: agonist Ab to TRAIL receptor DR4 and DR5, and TRAIL-armed CD34+ cells. Interestingly, agonist Ab to DR4, but not to DR5, were able to induce apoptosis in cells that showed DR4 but not DR5 associated with GM3, the main glycosphingolipid component of lipid rafts in lymphocytic cells.16 This finding is in agreement with the observation that protein/glycosphingolipid interaction within lipid rafts may have a key role in apoptotic pathway.17, 21
Some recent papers suggest that recruitment or localization of TRAIL receptors into lipid rafts can promote cancer cell death. In particular, it was reported that redistribution of DR4 and DR5 in lipid rafts accounts for the sensitivity to TRAIL of non-small cell lung carcinoma (NSCLC) cells.22 In other cell lines, lipid raft-dependent DR5 expression and activation are critical for ursodeoxycholic acid-induced apoptosis in gastric cancer cells,11 whereas DR4 recruitment into lipid rafts induced synergistic apoptotic response to fludarabine in Burkitt lymphoma B cells.23 Furthermore, the fact that nystatin, a cholesterol-sequestering agent, can partially prevent TRAIL receptor localization into lipid microdomains and consequently reduce TRAIL-induced apoptosis in NSCLC cells12 appears in agreement with our data indicating a significant reduction of TRAIL-induced apoptosis by using the raft disruptor MBC.
At variance, chemotherapic drugs bolstering TRAIL receptor recruitment into lipid rafts, for example, epirubicin,24 cisplatin25 or perifosine and edelfosine13 significantly increased TRAIL sensitivity, suggesting that the combination of these drugs with TRAIL might offer a novel therapeutic strategy for cancer. In line with this hypothesis, some recent literature insight proposes the use of agents that modify the composition and structure of lipid membranes as anticancer drugs.26 Interestingly, in all the above papers, in agreement with the results reported here, no significant positive correlation was found between expression levels of DR4 or DR5 and sensitivity to TRAIL. Thus, DR expression seems to be necessary but not sufficient for TRAIL sensitivity.
Our analyses suggest that the mechanism underlying TRAIL sensitivity is based on the association, within lipid microdomains, of DRs DR4 with a specific ganglioside, that is, the monosialoganglioside GM3. This was demonstrated by using FRET technique either in lymphoblastoma cell lines or in B lymphocytes freshly isolated from naïve patients affected by CLL. FRET is well known for its usefulness and handiness as valuable analytical cytology method to investigate molecular associations protein/protein as well as protein/(sphingo)lipid15, 27 by using a reduced amount of biological material in comparison to biochemical methods. In fact, FRET analysis allowed us to investigate the interaction of GM3 with TRAIL receptors also in B lymphocytes freshly isolated from pathological subjects, in which biochemical analyses were banished because of the small amount of cells obtained by drawing of a blood sample. In addition, FRET technique (‘validated' by canonic biochemical methods) also allowed the study of the GM3/DR4 molecular association in entire non-permeabilized B lymphocytes selected for their expression of CD19. Lipid rafts could act as catalytic microdomains, where TRAIL-associated death signals can take place easily. As the association with these structures was already suggested for other receptors associated with cell death signals, for example, CD95/Fas,28, 29 we can hypothesize that the differences detected in the panel of cell lines considered here could depend on the integrity and function of lipid microdomains. Finally, GM3 is considered here as paradigmatic as particularly abundant in lymphoid cells but we cannot rule out the possibility that gangliosides others than GM3 could have a role in determining TRAIL sensitivity in other cell types.
One of the possible mechanisms involved in lipid raft partitioning of receptors could be the attachment to these proteins of fatty acids, such as palmitic acid, that is, palmitoylation. For instance, it was reported that the integration/retention into lipid rafts of DR4 and of a receptor of the same molecular family of DR, that is, TNFR1, can be regulated by palmitoylation, which enhances molecular hydrophobicity and contributes to their membrane association and their signaling function.21, 30 By contrast, mechanistic studies also showed that glycosylation promoted DR ligand-induced receptor clustering in plasma membrane microdomains, an event that was associated with efficient DISC recruitment and caspase-8 activation.31, 32
Results obtained with B-cell lines were fully superimposable to those obtained in B lymphocytes freshly isolated either from patients affected by CLL or from healthy donors: only those lymphocytes displaying TRAIL receptor DR4 embedded in lipid microdomains were susceptible to TRAIL-mediated apoptosis (sTRAIL, DR4 agonist antibodies, CD34+TRAIL-armed), that is, in four out of six naïve patients analyzed. By contrast, the lymphocytes isolated from six healthy donors and from two out of six patients considered, in which no association of TRAIL receptor (neither DR4 nor DR5) was found by FRET analysis, were fully resistant to TRAIL. In addition, as FRET can be performed in a small number of cells, we can also consider the possibility that this approach could provide a useful predictive information to the physician as concerns the susceptibility to TRAIL-mediated therapy or the possibility to booster TRAIL effects by using specific drugs able to modulate TRAIL-receptor recruitment into lipid rafts (e.g. perifosine, edelfosine). In few words, the association GM3/TRAIL can be predictive of a susceptibility to therapeutic use of TRAIL ligation, whereas the lack of this association could avoid useless chemotherapeutic interventions.
In conclusion, although further studies will be needed to clarify these points and to understand how TRAIL receptor DR4 can be ‘directed' into lipid rafts, the present work clearly suggests a catalytic role of lipid microdomains and points at DR4 constitutively embedded in these structures as a putative predictive marker of TRAIL susceptibility for B lymphocyte malignancies.
Materials and Methods
Cells
Human cancer cell lines used included Namalwa, Raji and Daudi EBV-positive, Burkitt lymphoma cell lines; the Ramos EBV-negative Burkitt lymphoma cell line; CEM T-cell line and the CD30−/CD68+ HD-MyZ histiocytic cell line.33 Non-transformed cells included human PBL and skin fibroblasts isolated from six consenting healthy donors and human umbilical vein endothelial cells (HUVEC, PromoCell, Heidelberg, Germany, EU). Cells were maintained in RPMI 1640 medium or DMEM (both Gibco-BRL, Life Technologies Italia, Milano, Italy) supplemented with 10% fetal calf serum, at 37 °C in a humidified 5% CO2 atmosphere. Adenovirus-transduced CD34+ cells expressing membrane-bound TRAIL (CD34-TRAIL+ cells) were obtained as described elsewhere in more detail.19
CLL cells
Primary CLL mononuclear cells (MNCs) were isolated at the time of diagnostic work-up from the peripheral blood of six consenting patients by centrifugation on a Ficoll/Hypaque density gradient and plastic adherence to deplete monocytes. As assessed by flow cytometry, the percentage of MNCs co-expressing CD19, CD5 and CD23 antigens was >90%. B-CLL cell samples from naive patients were chosen as, with respect to lymphoma bioptic samples, they can represent a paradigmatic and valuable example of ex vivo freshly isolated B cells.
Treatments
Apoptosis was induced by incubating cells with recombinant soluble TRAIL (TRAIL 100 ng/ml for 48 h, Adipogen International, Inc. San Diego, CA, USA) or with agonist antibodies anti-DR4 and anti-DR5 (2 μg/ml, both R&D Systems, Minneapolis, MN, USA). To modulate rafts organization, cells were pretreated with 5 mM methyl-beta-cyclodextrin (MBC, a lipid raft disruptor that removes cholesterol from membranes, Sigma, St. Louis, MO, USA) for 30 min or 5 μM perifosine for 24 h (10 μM for ex vivo experiments), which stimulates the recruitment of DR4 and DR5 into lipid rafts.13
Adenoviral transduction of CD34+ cells
CD34+ cells were positively selected using the AutoMACS device (Miltenyi Biotec, Bergisch Gladbach, Germany) from the blood of consenting donors undergoing stem-cell mobilization. The cDNA for human TRAIL was purchased from the Riken BioResource Center. A replication-deficient adenovirus encoding the human TRAIL gene (Ad-TRAIL) expressed from the cytomegalovirus promoter was generated using standard methods as previously described. 34 The transduction protocol has been described elsewhere in more detail (see Supplementary Methods).35
In vitro activity of membrane-bound TRAIL
The biological activity of mTRAIL-expressing cells was evaluated by coculturing CD34-TRAIL cells and tumor target cells for 48 h (1 : 1 effector:target cell ratio) in the presence or absence of anti-TRAIL-neutralizing monoclonal antibodies (BD Pharmingen, Franklin Lakes, NJ, USA).
Surface expression of Apo2L/TRAIL receptors by flow cytometry analysis
Surface expression of DR4 and DR5 was quantified by flow cytometry after cell staining with monoclonal anti-human DR4-phycoerythrin (PE) and monoclonal anti-human DR5-allophycocyanin (APC) (both BD, San Diego, CA, USA). As negative controls, we used mouse IgG-PE and mouse IgG-APC.
Cell death assays
Quantitative evaluation of apoptosis was performed by flow cytometry after double staining using FITC-conjugated annexin V/Trypan blue (Eppendorf, Milan, Italy), which discriminates early apoptotic, late apoptotic and necrotic cells.
Sucrose gradient fractionation
Lipid raft fractions from cells were isolated as previously described.36, 37 Briefly, 2 × 108 cells were suspended in 1 ml of lysis buffer and the lysate was centrifuged for 5 min at 1300 × g to remove nuclei and large cellular debris. The supernatant fraction (post-nuclear fraction) was subjected to sucrose density gradient centrifugation, that is, the fraction was mixed with an equal volume of 85% sucrose (w/v) in lysis buffer (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA). The gradient was fractionated and 11 fractions were collected starting from the top of the tube. All steps were carried out at 0–4 °C.
FRET by flow cytometry
We applied FRET analysis by flow cytometry in order to study the molecular association of GM3 and DR4 or DR5. Briefly, cells were fixed with 0.1% paraformaldehyde and labeled with antibodies tagged with donor (PE) or acceptor (Cy5) dyes. GM3 staining was performed by using unlabeled mouse antibody (Seikagaku Corp., Tokyo, Japan) and a saturating amount of biotinylated anti-mouse IgM (Sigma) followed by Streptavidin-Cy5 (Amersham, Buckinghamshire, UK). DR4 and DR5 staining was performed by using specific polyclonal antibodies (R&D Systems, Minneapolis, MN, USA) and saturating amount of PE-labeled anti-goat (BD Pharmingen). In addition, lymphocytes freshly isolated from healthy donors or from patients with hematological malignancies were stained with anti-CD19 FITC-conjugated monoclonal antibodies (BD Pharmingen) and analyses were restricted to CD19-positive cells only. Samples were analyzed with a dual-laser FACScalibur flow cytometer (BD Biosciences, Heidelberg, Germany) as previously described.15 The FE was calculated according to the study by Riemann et al.38 using the formula:
FE=(FL3DA—FL2DA/a—FL4DA/b)/FL3DA, where A is the acceptor and D is donor, a=FL2D/FL3D and b=FL4A/FL3A. For further information see Supplementary Methods, Supplementary Files 1 and 2.
Immunoblotting analysis of fractions
All the fractions obtained as reported above were subjected to sodium-dodecyl sulfate polyacrilamide gel electrophoresis. The proteins were electrophoretically transferred onto polyvinilidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA, USA). Membranes were blocked with 5% defatted dried milk in TBS, containing 0.05% Tween-20, and probed with anti-DR4 monoclonal antibodies or anti-DR5 polyclonal antibodies.
Bound antibodies were visualized with HRP-conjugated anti-mouse IgG (Jakson Immunoresearch Laboratories, Baltimore Pike, MD, USA) or anti-goat IgG (Dako, Glostrup, Denmark) and immunoreactivity assessed by chemiluminescence reaction, using the ECL Western blocking detection system (Amersham). FLOT2 was stained using a goat polyclonal IgG antibody (A-16) (Santa Cruz Biotechnology, Santa Cruz, CA, USA)
Immunofluorescence analysis
Control and treated cells were fixed with 0.1% paraformaldehyde. Cells were incubated with anti-DR4 or anti-DR5 polyclonal antibodies (R&D Systems) for 1 h at 4 °C followed by AlexaFluor 594 conjugated anti-goat IgG (Life Technologies, Mo, Italy) for additional 30 min. After washings, cells were incubated for 1 h at 4 °C with anti-GM3 IgM (Seikagaku, Tokyo, Japan), followed by AlexaFluor 488-conjugated anti-mouse IgM (Life Technologies). All samples were counterstained with Hoechst 33342, mounted with glycerol–phosphate-buffered solution (2 : 1) and analyzed by using an Olympus fluorescence microscope equipped with a Zeiss CCD camera (Olympus Corporation, Tokyo, Japan).
Data analysis and statistics
All samples were analyzed with a FACSCalibur cytometer (BD) equipped with a 488 argon laser and 633 visible red diode laser. At least 50 000 events were acquired. For experiments in which we restricted our analyses to CD19-positive cells, 100 000 events were acquired. Data were recorded by a Macintosh computer using CellQuestPro Software (BD). Statistical analyses were performed by using Student's t-test for paired samples or non-parametric Anova test. All data reported were verified in at least three different experiments and reported as mean±S.D. P-values <0.05 were considered as statistically significant.
Acknowledgments
This work was supported in part by grants from the Ministry of Education, University and Research (Rome, Italy), the Ministry of Health (Ricerca Finalizzata 2008 and 2010 to CC-S and WM), the Italian Association for Cancer Research (MCO—9998) (AMG; CC-S and WM); Peretti Foundation (WM); PRIN project 2009, Sapienza University project 2012 (MS) and Arcobaleno ONLUS (WM).
Glossary
- APC
allophycocyanin
- CLL
chronic lymphoblastic leukemia
- DR
death receptor
- FE
FRET efficiency
- FLOT2
flotillin 2
- FRET
fluorescence resonance energy transfer
- GM3
monoganglioside 3
- HD
healthy donor
- HUVEC
human umbilical endothelial cell
- IVM
intensified video microscopy
- MBC
methyl-beta-cyclodextrin
- MNCs
mononuclear cells
- PBL
peripheral blood lymphocytes
- mTRAIL
membrane-bound TRAIL
- PE
phycoerythrin
- sTRAIL
soluble TRAIL
- TNF
tumor necrosis factor
- TRAIL
tumor necrosis factor-related apoptosis-inducing ligand
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Author Contributions
MM performed the majority of experiments, data collection and analysis and final approval of the manuscript. BA, LC, TG, LSL and RV performed experiments, collected and analyzed the data and approved the manuscript. AMG and MS contributed to designing the experiments, writing the manuscript and fund raising. CC-S provided reagents, performed data analysis, contributed to the manuscript writing, fund raising and final approval of the manuscript. WM and PM conceptualized and designed the study, performed experiments, data analysis, manuscript writing, fund raising and assisted in the final approval of the manuscript.
Edited by G Raschellá
Supplementary Material
References
- Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Activation of apoptosis by Apo-2 ligand is independent of FADD but blocked by CrmA. J Biol Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002;2:420–430. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- Falschlehner C, Emmerich CH, Gerlach B, Walczak H. TRAIL signalling: decisions between life and death. Int J Biochem Cell Biol. 2007;39:1462–1475. doi: 10.1016/j.biocel.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Gajate C, Del Canto-Janez E, Acuna AU, Amat-Guerri F, Geijo E, Santos-Beneit AM, et al. Intracellular triggering of Fas aggregation and recruitment of apoptotic molecules into Fas-en- riched rafts in selective tumor cell apoptosis. J Exp Med. 2004;200:353–365. doi: 10.1084/jem.20040213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorice M, Matarrese P, Manganelli V, Tinari A, Giammarioli AM, Mattei V, et al. Role of GD3-CLIPR-59 association in lymphoblastoid T cell apoptosis triggered by CD95/Fas. PLoS One. 2010;5:8567. doi: 10.1371/journal.pone.0008567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma R, Mayor S. GPI-anchored proteins are organized in submicron domains at the cell sur- face. Nature. 1998;394:798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- George KS, Wu S. Lipid raft: a floating island of death or survival. Toxicol Appl Pharmacol. 2012;259:311–319. doi: 10.1016/j.taap.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min Y, Shi J, Zhang Y, Liu S, Liu Y, Zheng D. Death receptor 5-recruited raft components contributes to the sensitivity of Jurkat leukemia cell lines to TRAIL-induced cell death. IUBMB Life. 2009;61:261–267. doi: 10.1002/iub.166. [DOI] [PubMed] [Google Scholar]
- Lim SC, Duong HQ, Choi JE, Lee TB, Kang JH, Oh SH, et al. Lipid raft-dependent death receptor 5 (DR5) expression and activation are critical for ursodeoxycholic acid-induced apoptosis in gastric cancer cells. Carcinogenesis. 2011;32:723–731. doi: 10.1093/carcin/bgr038. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Yang C, Liu Y, Xiong J, Zhang J, Zhong Y, et al. Redistribution of DR4 and DR5 in lipid rafts accounts for the sensitivity to TRAIL in NSCLC cells. Int J Oncol. 2011;39:1577–1586. doi: 10.3892/ijo.2011.1129. [DOI] [PubMed] [Google Scholar]
- Gajate C, Mollinedo F. Edelfosine and perifosine induce selective apoptosis in multiple myeloma by recruitment of death receptors and downstream signaling molecules into lipid rafts. Blood. 2007;109:711–719. doi: 10.1182/blood-2006-04-016824. [DOI] [PubMed] [Google Scholar]
- Garofalo T, Misasi R, Mattei V, Giammarioli AM, Malorni W, Pontieri GM, et al. Association of the death-inducing signaling complex with microdomains after triggering through CD95/Fas. Evidence for caspase-8–ganglioside interaction in T cells. J Biol Chem. 2003;278:8309–8315. doi: 10.1074/jbc.M207618200. [DOI] [PubMed] [Google Scholar]
- Sorice M, Matarrese P, Tinari A, Giammarioli AM, Garofalo T, Manganelli V, et al. Raft component GD3 associates with tubulin following CD95/Fas ligation. FASEB J. 2009;23:3298–3308. doi: 10.1096/fj.08-128140. [DOI] [PubMed] [Google Scholar]
- Xing Y, Gu Y, Xu LC, Siedlecki CA, Donahue HJ, You J. Effects of membrane cholesterol depletion and GPI-anchored protein reduction on osteoblastic mechanotransduction. J Cell Physiol. 2011;226:2350–2359. doi: 10.1002/jcp.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KW, Punnoose EA, Januario T, Lawrence DA, Pitti RM, Lancaster K, et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med. 2007;13:1070–1077. doi: 10.1038/nm1627. [DOI] [PubMed] [Google Scholar]
- Wajant H, Moosmayer D, Wüest T, Bartke T, Gerlach E, Schönherr U, et al. Differential activation of TRAIL-R1 and -2 by soluble and membrane TRAIL allows selective surface antigen-directed activation of TRAIL-R2 by a soluble TRAIL derivative. Oncogene. 2001;20:4101–4106. doi: 10.1038/sj.onc.1204558. [DOI] [PubMed] [Google Scholar]
- Lavazza C, Carlo-Stella C, Giacomini A, Cleris L, Righi M, Sia D, et al. Human CD34+ cells engineered to express membrane-bound tumor necrosis factor-related apoptosis-inducing ligand target both tumor cells and tumor vasculature. Blood. 2010;115:2231–2240. doi: 10.1182/blood-2009-08-239632. [DOI] [PubMed] [Google Scholar]
- MacFarlane M, Inoue S, Kohlhaas SL, Majid A, Harper N, Kennedy DB, et al. Chronic lymphocytic leukemic cells exhibit apoptotic signaling via TRAIL-R1. Cell Death Differ. 2005;12:773–782. doi: 10.1038/sj.cdd.4401649. [DOI] [PubMed] [Google Scholar]
- Poggi M, Kara I, Brunel JM, Landrier JF, Govers R, Bonardo B, et al. Palmitoylation of TNF alpha is involved in the regulation of TNF receptor 1 signalling. Biochim Biophys Acta. 2013;1833:602–612. doi: 10.1016/j.bbamcr.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Yang C, Zhang S, Liu Y, Yang B, Zhang J, et al. Absence of death receptor translocation into lipid rafts in acquired TRAIL-resistant NSCLC cells. Int J Oncol. 2013;42:699–711. doi: 10.3892/ijo.2012.1748. [DOI] [PubMed] [Google Scholar]
- Xiao W, Ishdorj G, Sun J, Johnston JB, Gibson SB. Death receptor 4 is preferentially recruited to lipid rafts in chronic lymphocytic leukemia cells contributing to tumor necrosis related apoptosis inducing ligand-induced synergistic apoptotic responses. Leuk Lymph. 2011;52:1290–1301. doi: 10.3109/10428194.2011.567317. [DOI] [PubMed] [Google Scholar]
- Xu L, Qu X, Luo Y, Zhang Y, Liu J, Qu J, Zhang L, Liu Y. Epirubicin enhances TRAIL-induced apoptosis in gastric cancer cells by promoting death receptor clustering in lipid rafts. Mol Med Rep. 2011;4:407–411. doi: 10.3892/mmr.2011.439. [DOI] [PubMed] [Google Scholar]
- Vondálová Blanárová O, Jelínková I, Szöor A, Skender B, Soucek K, Horváth V, et al. Cisplatin and a potent platinum(IV) complex-mediated enhancement of TRAIL-induced cancer cells killing is associated with modulation of upstream events in the extrinsic apoptotic pathway. Carcinogenesis. 2011;32:42–51. doi: 10.1093/carcin/bgq220. [DOI] [PubMed] [Google Scholar]
- Llado V, Gutierrez A, Martínez J, Casas J, Terés S, Higuera M, et al. Minerval induces apoptosis in Jurkat and other cancer cells. J Cell Mol Med. 2010;14:659–670. doi: 10.1111/j.1582-4934.2008.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Dueñas V, Llorente J, Gandía J, Borroto-Escuela DO, Agnati LF, Tasca CI, et al. Fluorescence resonance energy transfer-based technologies in the study of protein-protein interactions at the cell surface. Methods. 2012;57:467–472. doi: 10.1016/j.ymeth.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Lang I, Fick A, Schäfer V, Giner T, Siegmund D, Wajant H. Signaling active CD95 receptor molecules trigger co-translocation of inactive CD95 molecules into lipid rafts. J Biol Chem. 2012;287:24026–24042. doi: 10.1074/jbc.M111.328211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert S, Loranger A, Lavoie JN, Marceau N. Cytoskeleton keratin regulation of FasR signaling through modulation of actin/ezrin interplay at lipid rafts in hepatocytes. Apoptosis. 2012;17:880–894. doi: 10.1007/s10495-012-0733-2. [DOI] [PubMed] [Google Scholar]
- Rossin A, Derouet M, Abdel-Sater F, Hueber AO. Palmitoylation of the TRAIL receptor DR4 confers an efficient TRAIL-induced cell death signalling. Biochem J. 2009;419:185–192. doi: 10.1042/BJ20081212. [DOI] [PubMed] [Google Scholar]
- Wagner KW, Punnoose EA, Januario T, Lawrence DA, Pitti RM, Lancaster K, Lee D, von Goetz M, Yee SF, Totpal K, Huw L, Katta V, Cavet G, Hymowitz SG, Amler L, Ashkenazi A. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med. 2007;13:1070–1077. doi: 10.1038/nm1627. [DOI] [PubMed] [Google Scholar]
- Klíma M, Zájedová J, Doubravská L, Andera L. Functional analysis of the posttranslational modifications of the death receptor 6. Biochim Biophys Acta. 2009;1793:1579–1587. doi: 10.1016/j.bbamcr.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Locatelli SL, Giacomini A, Guidetti A, Cleris L, Mortarini R, Anichini A, et al. Perifosine and sorafenib combination induces mitochondrial cell death and antitumor effects in NOD/SCID mice with Hodgkin lymphoma cell line xenografts. Leukemia. 2013;27:1677–1687. doi: 10.1038/leu.2013.28. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Yamaguchi N, Nakayama M, Kawasaki A, Akiba H, Okumura K, et al. Involvement of TNF-related apoptosis-inducing ligand in human CD4+ T cell-mediated cytotoxicity. J Immunol. 1999;162:2639–2647. [PubMed] [Google Scholar]
- Lavazza C, Carlo-Stella C, Di Nicola M, Longoni P, Milanesi M, Magni M, et al. Highly efficient gene transfer into mobilized CD34+ hematopoietic cells using serotype-5 adenoviral vectors and BoosterExpress Reagent. Exp Hematol. 2007;35:888–897. doi: 10.1016/j.exphem.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Garofalo T, Lenti L, Longo A, Misasi R, Mattei V, Pontieri GM, et al. Association of GM3 with Zap-70 induced by T cell activation in plasma membrane microdomains: GM3 as a marker of microdomains in human lymphocytes. J Biol Chem. 2002;277:11233–11238. doi: 10.1074/jbc.M109601200. [DOI] [PubMed] [Google Scholar]
- Malorni W, Garofalo T, Tinari A, Manganelli V, Misasi R, Sorice M. Analyzing lipid raft dynamics during cell apoptosis. Methods Enzymol. 2008;442:125–140. doi: 10.1016/S0076-6879(08)01406-7. [DOI] [PubMed] [Google Scholar]
- Riemann D, Tcherkes A, Hansen GH, Wulfaenger J, Blosz T, Danielsen EM. Functional co-localization of monocytic aminopeptidase N/CD13 with the Fc gamma receptors CD32 and CD64. Biochem Biophys Res Commun. 2005;331:1408–1412. doi: 10.1016/j.bbrc.2005.04.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.